HYLE--International Journal for Philosophy of Chemistry, Vol. 4 (1998), No. 1, pp. 3-27
Abstract: A historical overview of the development of chemical signs reveals the central role of the Table as a representational device, as well as its limitations. Furthermore, the decreasing importance of linguistic signs such as names, compared to iconic signs such as structural formulas, accords with and reinforces the intensely visual character of chemistry. Chemistry's symbolic language is shown to mimic many features of natural languages, including the ability to construct fictional worlds. I argue that these 'scientific fictions' are as cognitively valuable in chemistry as they are in ordinary life, and that chemists creatively mix 'true' and 'fictional' representations of molecules and substances.
Keywords: fictional science, maps, metaphors, representation, semiology, tables.
When a finger points at the moon,
only an idiot looks at the finger.
- Chinese aphorism -
In what follows I will play the idiot but not, I hope, the fool by giving as much attention to signs as to objects. My focus will be on microscopic objects, atoms and molecules, and on the signs by which chemists refer to them. Among other questions, I want to ask whether the relations between those signs and those objects are the same as the transparent relations that are assumed to hold between moons and pointing fingers. It is, after all, a near-universal belief that celestial bodies like the moon antedate and are independent of humans, and that people pointing at the moon are merely using a conventional sign to designate an independent object. Are chemical signs and chemical objects similarly independent of one another?[1]
Until recently, the conventional view has been that scientific languages are fundamentally different from ordinary language: scientific languages are often characterized as univocal, stripped of connotations and implicit meanings, passive and transparent (Carlisle 1980). However, studies of the rhetorical content of scientific languages have shown that they are far from meeting that ideal (Jordanova 1986, Schuster and Yeo 1986).
Nonetheless, it is still possible to adhere to the 'received view' in a modified form by recognizing that, broadly speaking, scientific languages may be divided into 'informal' and 'formal' discourses. One can allow that the informal discourse, based as it is almost entirely on ordinary language, displays virtually all the characteristics of that language. These encompass multiple and implicit meanings and references, emotive qualities, rhetorical devices, metaphorical structures, and so on. Nonetheless, a sharp boundary is alleged to separate informal scientific discourse from its formal counterpart which, by relying heavily on nonverbal symbols, presumably escapes the 'traps' of ordinary language and approaches the neutral, transparent ideal referred to above.
The prevalent notion of a wide separation - a wall, a chasm, a cordon sanitaire - between formal scientific language and natural language is succinctly put by Guiraud (1975, pp. 27-28):
Denotation and connotation constitute two fundamental yet opposed modes of signification The sciences belong to the denotative type, and the arts to the connotative In theory, for communication to be effective, for each signified there should be one signifier and one only, and vice versa. This is the case for scientific languages, signalling systems and logical codes in general
But language, like life, is rarely so straightforward. Scientific languages cannot stand in splendid isolation; as Wilda Anderson observes about chemical nomenclature, "To function, it must be embedded in natural language" (Anderson 1985, 168). Furthermore, although we may classify the discourses of a science as either 'informal' or 'formal', there are in fact a plurality of discourses within any scientific language (Mestrallet 1981, pp. 8-10). They form a continuum from those that consist almost entirely of ordinary language to those based totally on technical terms and non-verbal symbols. What's more, the structure of the chemical symbol system may even mimic that of ordinary language in a large number of ways (vide infra). For all these reasons, I believe it plausible to approach the language of chemistry just as we would any other language. Rather than analyze scientific signs and discourse in the abstract, my intent is to show how a semiotic analysis of chemical representation can shed light on the following topics:
My focus will be primarily on the universe of signs; a fully social semiotic analysis has yet to be written.
The representational systems of chemistry have been the object of continuing investigation for reasons that will, I hope, be readily apparent. An indispensable historical overview is provided by Maurice Crosland, whose primary concern is the linguistic components (Crosland 1962). If his book somewhat slights the enormously important (and increasingly dominant) symbolic representations (Wightman 1963, p. 261), that lack is amply supplied by the comprehensive semiological study of chemist-turned-linguist Renée Mestrallet (Mestrallet 1980, p. 1981). In contrast to Crosland her approach is synchronic rather than historical, her perspective semiotic rather than chemical. Mary Jo Nye has carefully examined chemical symbols within a larger discussion of chemical theory (Nye 1993), and some recent explorations by Roald Hoffmann and Pierre Laszlo reflect the insights of two prominent, active practitioners (Hoffmann and Laszlo 1991, Laszlo 1993, Hoffmann 1995).
Unlimited as they are, the languages of chemistry - formal and informal, iconic and linguistic - do not exhaust the disciplinary repertoire of representations. The table is also a ubiquitous form of chemical representation, one that features prominently in Dagognet's philosophical and poetic meditation on chemistry. According to him, the paradigmatic example of that genre, the Periodic Table, was
nothing less than the means of photographing the entire chemical family, [making] a map of the whole group of material continents, in other words, a classification at once rational and complete chemists have brought off the most amazing masterstroke, that of being able to outline - in the famous Table - the totality of materials, with the properties of each one and the multiple links which bind it to the others. With very few signs and a few columns they have been able to assemble not only the real in all its variety and plenitude, but the unknown and the nonexistent as well. (Dagognet 1969, p. 10; emphasis added)
The metaphor of the map is a recurrent one in chemical contexts, used by both chemists and commentators. In the synoptic table of solvent activity assembled by Guyton de Morveau and his colleagues in 1777 we see "as upon the grid of a world map, the lands we have yet to discover" (Roberts 1991, p. 114). A decade later Guyton joined Lavoisier, Fourcroy and Berthollet to promulgate a new nomenclature that "would mark in advance the place and the name for new substances that it will be possible to discover" (Guyton 1787, p. 17). After two centuries the metaphor was still apposite: "The table [of Geoffroy] is a map of the whole area of operations that could be reversed a coherent map of displacement reactions was produced that made a quick overview of the whole ordered field possible." (Klein 1996, pp. 276, 278)
The word 'map' in turn activates other metaphors, such as discovery. As chemists' focus moved gradually from extraction and analysis to reaction and synthesis, the meaning of 'discovery' expanded and its center of gravity was displaced, without any explicit notice being taken. 'Map' also entails the concept of dimension, and dimension is a parameter that applies to signs as well as to things (Bunn 1981). One purpose of this paper is to draw attention to the parallel expansion of molecular conceptions and representational devices, of excursions into physical space and semiotic space.
It is pertinent to recall that maps are anything but passive. They may summarize, they may describe, but they also direct and inscribe (Thongchai 1994). Chemical maps present this Janus face as well, simultaneously revealing and concealing. My hope is to uncover a part of the physiognomy that lies in the shadows.
Geoffroy's Table des rapports of 1718 was the first of these topological constructs to profoundly shape the organization, interpretation and presentation of chemical information (Figure 1). Each of its columns is headed by the symbol for a substance or class of substances, followed by symbols representing a group of reactants that combine with it. The reactants are arranged in order of decreasing affinity for the substance(s) in question. The symbols, many of them borrowed from alchemy, are for the most part labels - they designate the materials without telling us anything about their composition. In fact, reaction rather than composition is the subject of this table, which is not about substances but rather about their transformations.
Figure 1. Geoffroy's Table des rapports (Geoffroy [1718]).
A casual glance might suggest that Geoffroy's table does little more than summarize a body of empirical data, displaying the relative reactivities of members of various chemical groups toward one another (Roberts 1991, pp. 105-6). However, behind this facade of ingenuous empiricism lies a network of theory, much of it covert. The substances are treated not only individually but also as groups, which already supposes a method of classification. Furthermore, there is an attempt to account for the order of relative reactivities by invoking what Geoffroy calls "a disposition to unite" or "a relationship of union". That this "disposition to unite" can indeed function as a causal explanation is shown by its efficacy in demonstrating why and how different preparations of corrosive sublimate [mercury(II) chloride] all give rise to the same substance (Geoffroy [1718], pp. 316-19).
However, the causes invoked are proximate rather than ultimate ones. For Geoffroy and his contemporaries any ultimate cause would had to have been grounded in Newtonian mechanics, and numerous attempts to produce Newtonian explanations of characteristic chemical phenomena, such as the differential affinities displayed in Geoffroy's table, were unconvincing. While a practice without a theory could never attain the status of a science, too speculative a theory would raise the ghost of alchemical mysticism. Geoffroy's "disposition to unite", tucked away in the interstices of his table, eluded the pitfalls of either (and also gave a preview of the wary relationships between physicists' and chemists' theories in the centuries to come). Geoffroy's focus on reactions held at bay the claims of the mechanical philosophy and provided a model of an indigenous chemical theory. Yet the pull of mechanical explanations could never be totally ignored: "Since Geoffroy chemistry has wavered between two problematics: a science of matter or a science of reactions." (Guédon 1980, p. 103)
Topologically, Geoffroy's table is one-dimensional. Each column is to be read vertically and independently of the others. There is no coherent horizontal reading of the table. In Guédon's words, the results were deployed "in the virtual space of a table that took account of the reactions in order to rearrange them hierarchically so far as possible" (Guédon 1980, p. 108). The columns rather resemble medieval maps showing travelers the stages of the pilgrimage to Jerusalem (Brown 1949, pp. 97-102). Similar maps were produced locally in southeast Asia; Thongchai (1994, p. 31) describes one as "a map of a piece of the earth's surface similar to a modern map. But unlike a modern map, it does not yet show how such a piece of the earth's surface relates to, or is situated on, the globe". Chemists would only acquire such a God's eye view at the end of the 18th century.
Historians are of several minds regarding the extent to which Geoffroy's achievement represents a sharp break with those of his predecessors (Roberts 1991, Holmes 1996, Klein 1996). Yet there seems little doubt that it established the table as a paradigmatic organizational device in chemistry. Furthermore, to a greater or lesser degree Geoffroy's table - with all that it implied - adumbrated many of the themes and controversies that would occupy chemists up to the present day. For example, the distinction between the 'natural' and the artifactual was being steadily undermined; with respect to mineral substances it was fast eroding (Klein 1996, p. 274).
This erasure of boundaries was consequential in a number of ways. The Table des rapports presupposed a method of classification, but that classification rested in turn on exactly the sorts of operations summarized in the table: " the identification of natural bodies within chemistry was done by intervening in the constitution of natural bodies with chemical tools." (Klein 1996, p. 281) The very same tools that were used in the classification of known bodies could also be used in the synthesis of new ones, as chemists increasingly manufactured that which they studied. The chemist and nature became less and less distinguishable; the discoverer would soon become one with the discovered.
Stimulated in part by the conception of affinity itself and propelled by the momentum of established traditions of productive practice, chemists generated and classified a rapidly growing body of substances (Holmes 1989, Klein 1994). Tables of affinity were indispensable for bringing order to this proliferation, and the table became firmly entrenched as the frame of chemical knowledge. However, its function was to change dramatically in the course of the 18th century (Roberts 1991).
Toward the end of that century Lavoisier used the potentialities of the table to promulgate two inseparable and irreversible directions for chemistry. They were based on linguistic and experimental developments already underway. The Linnaean system of botanical classification provided an important precedent for the binary naming system advocated by Lavoisier and his colleagues in the Méthode de nomenclature chymique of 1787 (Crosland 1962, pp. 139-143; Paradis 1983). And the salts to which that binary nomenclature was so successfully applied were themselves the products of a research program that had stretched over a century (Holmes 1989, pp. 33-59). It was the manner in which these developments were extended and linked that gave Lavoisier's initiatives their great impact.
The chemical revolution encompassed a complex interplay of continuities and discontinuities that the semiotic system both illuminated and concealed. For example, the Linnaean system may have served as the model for the new nomenclature, but as Wightman (1963, p. 264) shrewdly observed,
the chemical substances to be renamed constitute a class of objects very different from botanical species. The latter were, for Linnaeus, as God had created them in the beginning; the former are 'created' by the chemist So, as Lavoisier fully recognized, there could be no reliable nomenclature except on the basis of 'reliable' theory.
Lavoisier's 1789 textbook, Traité élémentaire de chimie, revealed "the full implications of collapsing natural knowledge into manipulative practice" (Roberts 1991, p. 123). The nomenclatural reform swept away the residue of uninformative and misleading names that increasingly obstructed the flow of chemical communication. From the perspective of the reformers their most serious defect had been that they referred arbitrarily to one or another property of the substance - provenance, appearance, medicinal use, etc. Set out in 48 tables, the new names were composed of two terms, derived from the acid and the base, respectively, whose combination gives rise to the salts (which constituted by far the largest category of substances). Each table is headed by an acid beneath which lie the names of bases with which that acid reacts, arranged according to affinity. Acids from the animal, vegetable and mineral kingdoms, 'natural' and synthetic, are placed side by side without any qualitative distinction being made among them.
All the oxyds and acids from the animal and vegetable kingdoms are formed from a small number of simple elements We may justly admire the simplicity of the means employed by nature to multiply qualities and forms We shall find the means no less simple and diversified, and as abundantly productive of forms and qualities, in the order of bodies we are about to treat of. (Lavoisier 1790, p. 199)
The walls between nature and art were being breached yet further, and in the process Lavoisier naturalized his own system of classification and denomination, and "establish[ed] composition as the fundamental organizing principle for all chemical knowledge" (Siegfried 1982, p. 46). Affinity was temporarily overshadowed and chemistry edged closer to being a science of matter.[2]
The distance that separates Lavoisier from Geoffroy is less discernible in the textbook's tables of salts than in its Table of Simple Substances (Lavoisier 1790, pp. 175-6, Figure 2). This is not merely one table among many; it is primus inter pares. By placing caloric and oxygen among the most ubiquitous of the simple substances Lavoisier was clearly reifying his theories of phase change, combustion and acidity. However, it is the definition of 'simple substance' that marks his most radical break with the past. Lavoisier rejects alike the outmoded metaphysics of the four elements - "a prejudice which has descended to us from the Greek philosophers" - and the untestable hypotheses of the mechanical philosophy - "it is extremely probable that we know nothing [of] those simple and invisible atoms of which matter is composed." However, he will admit as elements "all the substances into which we are capable, by any means, to reduce bodies by decomposition" (Lavoisier 1790, p. xxiv). Chemistry may be veering toward a science of matter but its foundations will rest on chemical criteria.[3]
Figure 2. Table of Simple Substances (Lavoisier 1790).
Lavoisier's program, indebted as it was to the Logique of Condillac, was self-consciously explicit about its intentions.
A well-formed language will not allow those who profess chemistry to diverge from the march of nature. They will either have to reject the nomenclature or else to follow irresistibly the route that it will have marked out [The language] will naturally adapt itself to the work to be done. It will mark in advance the place and the name for the new substances that it will be possible to discover (Guyton 1787, pp. 12, 16-17; emphasis added)
The import here is not only that new substances will have names that are logical and consistent with those already present; it is also that the categories of what could possibly be known have already been prefigured by the nomenclature. In other words, the system will not only provide a 'pigeonhole' for the products of future operations, it will constrain the interpretation of those operations (Anderson 1984, pp. 116-127).
In this way the nomenclature indeed created 'a grid of a world map'. We may glimpse a fragment of that map by laying side by side several tables from the Elements (Figure 3). It is evident that there are gaps here; also evident is sort of information that will be supplied to fill those gaps. New bases and acids would undoubtedly be discovered; relative affinities would unquestionably be altered - no matter, the system will accommodate these changes and many more.
Figure 3. Several Tables of Acid Salts, laid side by side to form a 'grid' (Lavoisier 1790).
This Lavoisian grid is reminiscent of a Mercator projection. Early Mercator maps were characterized by large areas of terra incognita, and the adventurers who attempted to fill them in encountered many stunning surprises. Yet, those explorers knew what sorts of topological features they could expect - mountains, islands, rivers. And they could be assured that they would not sail off the edge of the earth and perish. Generations of cartographers have deplored the distortions and inadequacies of Mercator's projection; nonetheless, it persists. The Lavoisian nomenclature has similarly been amended, revised, excused; yet its fundamental core is still with us.
Despite the difference in scope, intention and dimensionality between the tables of Geoffroy and Lavoisier, they share several important features. Both are closely tied to concrete material operations. For the experienced practitioner, seeing the sign of the substance, whether formed from linguistic (Lavoisier) or non-linguistic (Geoffroy) symbols, is to grasp almost immediately the route for preparing it; there is a congruence of sign and action. Above all, the table as visual display echoes the intense visuality of chemistry itself, elevating style to the level of substance. Assessing the most ubiquitous successor to the tables of Geoffroy and Lavoisier, Nye (1993, p. 89) writes:
Here is a scheme [the Periodic Table] which is an explanatory and predictive model and an icon in both the semiotic and the popular senses of the word. But its power comes from visual display, from image, not the principles and facts which can be recorded in ordinary or conventional language.
It is no accident that glass continues to be the preferred medium for laboratory vessels. Visual contact between chemist and substrate is a sine qua non even now when instruments are so indispensable to chemical science.[4] Analogously, the reformed nomenclature in Lavoisier's tables strove to exemplify Enlightenment ideals of semiotic transparency:
One must avoid everything through which the attention is diverted from the object itself and is steered principally toward the contemplation of the signs and images in which the object is clothed. (Meier [1718-1777], p. 52)
The upheavals to come in the following century would be marked semiologically by an increasing preoccupation with signs themselves, and by a weakening of the intimate reciprocity between sign and experiment.
Lavoisier's oxygen theory of acidity has long been abandoned along with his notions of the materiality of heat. Yet his impact on the development of chemistry has been unquestionably profound and nowhere more so than through his recasting of chemical language. Despite extensive alterations, the binary nomenclature is still in use, axiomatically tied to a binary theory of combination that explains the cohesion of a salt on the basis of opposite electrical charges on the two constituents designated in the name. At the time that the nomenclature was invented no such theory had been conceived. But when chemical combination was shown to have an electrical basis early in the 19th century, the binary nomenclature provided a perfect matrix for its theoretical elaboration.
True to Lavoisier's hopes the binary sign dominated the thinking of chemists decades after its ascendancy. Fittingly, its limitations were exposed by precisely that area of chemical investigation that was most peripheral to his reforms. The isolation of substances from animal and plant tissue, so-called organic materials, has an extensive history; one of its principal motivations had been the winning of medicinally active components (Holmes 1989, pp. 68-83). These substances, rather than incorporating the full panoply of known elements, seemed restricted to a paltry few - carbon and hydrogen always, oxygen very frequently, nitrogen often and a few others only occasionally. A large fraction of them were liquids and usually isolated in the company of similar materials from which they were separated with difficulty, if at all. Even in their pure state these compounds underwent transformations that often could not be 'read' within the prevailing semiotic system - precipitates were uncommon, dependable color changes even more so, reversible processes rarer yet.
Attempts to bring these substances and their reactions within the compass of binary combination failed. The electrochemical dualism that accounted so successfully for the cohesion of inorganic compounds seemed irreconcilable with a number of empirical observations. This theory and its rival, the theory of types, were both grounded in an implicit axiom of the Geoffroy/Lavoisier tables, that the reaction products were already present as cohesive components of the reactants. Neither theory was able to make the sense of the entire corpus of organic chemistry. Half a century after the triumph of Enlightenment discipline the 'march of nature' seemed to be leading chemists into a wilderness.
Even now the profoundest chemical philosophers of the age are contending for two theories respecting chemical combination, which are not at all similar to each other we discarded the idea we at first conceived of attempting a scientific arrangement of the substances we have presented The discoveries of the homologous series of bodies will enable us, perhaps, to give the vast mass of chemical facts so lately discovered, 'a habitation and a name'; to arrange them in such a correlated series as will accord with the great scheme of the Creator in the building up of the thousands of organic forms so prolifically strewn about us. (Sanders, in: Gregory 1860, pp. 7-8; emphasis added)
Lacking as they did the characteristics needed for inclusion in the Lavoisian system, most organic compounds lacked generally recognized systematic names as well. Recourse was often had to the old categories - origin, taste, odor - to designate them. The only sure method of characterizing these substances was by elemental composition, which depended in turn on accurate quantitative analyses. That was some decades in coming, but its realization further stimulated the laboratory creation of new organic compounds (Kim 1992, p. 69).
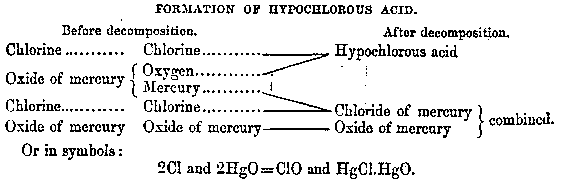
Expressing the results of chemical analyses in compact and unequivocal form therefore became a priority for chemists, resulting in the introduction of symbols to stand for the elements (Crosland 1962, p. 280). As with all modifications of chemical language, this initiative was contested, but Berzelius' 1814 proposal for an alphabetic system eventually won universal acceptance. It involved using one or two alphabetic characters derived from an element's Latin name to stand for that element. But what precisely did these symbols represent?
Wightman asserts that combining these letters with numbers "changed the status of 'symbols' in the restricted sense from mere abbreviations into the elements of an 'algebra' and later a 'geometry' or 'topology'" (Wightman 1963, p. 266). However, compared to the Lavoisian ideal this system was quite undisciplined - it allowed a broad range of usages and interpretations reminiscent of a natural language (Mestrallet 1981, pp. 10-12). Formulas based on alphabetic characters did sometimes function as little more than abbreviations (Figure 4). Moreover, because the symbols are letters of the alphabet they are inescapably tied to the linearity of reading and writing; to become truly topological the semiotic system would have to minimize its reliance on letters. Furthermore, the precise referent of these symbols was ambiguous. They could be seen as standing for nothing more than the number of combining weights of each element in the compound. However, when juxtaposed in particular ways within so-called rational formulas, the symbols suggested the proximity of atoms in the molecules and/or their correlated behavior during chemical transformations. Gerhardt saw this last possibility as the only worthwhile function of formulas, famously declaring that there could be "as many rational formulas as there are reactions" (Crosland 1962, p. 331). "With this view, the rigid connection between theory, formulas and nomenclature was abandoned, and arbitrariness was elevated to the status of a principle." (Priesner 1989, p. 224)

Figure 4. Chemical formulas as abbreviations in reaction equations.
Chemists also struggled to retain synoptic tables as the organizational framework for the rapidly accumulating discoveries in organic chemistry (Figure 5), but this form of representation was being stretched beyond its limits. The principal problem was not the number of compounds requiring classification; it was rather the nature of the relationship among the substances to be classified. Entire families of organic compounds emerged that differed compositionally to only a small extent from neighboring ones, as illustrated for the hydrocarbon family based on the generic formula CnH2n and its derivatives (Figure 6; Miller 1869, pp. 38-39). One can imagine an entirely analogous table for, say, the CnH(2n-2) family. To arrive at a coherent ordering of all these substances one would lay the tables not side by side, as with Lavoisier's, but rather one atop another to create a three-dimensional lattice. Indeed, contemporaneous chemists recognized three relational dimensions: compounds within a single table were related homologously in the vertical direction and collaterally in the horizontal one. And above and below each compound in a table, even if "for the most part, imaginary", lay its isologues (Miller 1869, pp. 32-41, 166-168). To return to our map metaphor, it was as if the oceans were beginning to recede, revealing beneath the surface of the earth previously unimagined continents and thus exposing the limitations of the Mercator projection.
Figure 5. Homologous hydrocarbon 'radicals' and some derivatives (Gregory 1860).
Figure 6. Homologous of CnH2n and their collateral 'heterologues' (Miller 1867).
The tables of Figures 5 and 6 illustrate another break with the Lavoisian past. In the ancien regime oxides of various kinds took pride of place in the tables of compound substances, but in Miller's tables hydrides rule, most specifically the hydrocarbons. Unlike their inorganic predecessors, organic tables were no longer capable of showing transparently the operations required to create their subjects. Many organic transformations do not precede in a single step and/or are not reversible. For example, to convert an alkene (a compound with a carbon-carbon double bond) into an alkane (a compound with only carbon-carbon single bonds) requires only one step. However, the reverse transformation normally requires two steps and passes through an intermediate compound (true even today). The net result was that locating a compound within the lattice of composition did not automatically reveal the recipe for its preparation.
Clearing a path through this 'jungle' of 19th-century organic chemistry meant using half-understood reactions to gain clues about structure, and tentative structural hypotheses to make sense of the reactions. As Biot noted, " [chemistry] only judges bodies after they no longer exist" (Biot 1854, vi). In other words, chemical practice itself is a semiotic exercise, one built around natural signs.
Enlightenment philosophers recognized that natural phenomena could be understood semiotically - for example, clouds as a sign for rain, rain as a sign for spring, and so on. They assumed that there was a qualitative distinction between 'natural' and conventional signs; I, however, agree with Greimas (1987, pp. 17, 19) that
the only conceivable presence signification can take on in the world is through its manifestation in the 'substance' surrounding human beings. From this perspective the sensible world as a whole becomes the object of the quest for signification it is necessary to postulate the existence and the possibility of a semiotics of the natural world and to think of the relation between 'natural' signs and natural languages, on the one hand, and signs and systems of signification of the natural world, on the other, not as reference stemming from the symbolic to the natural, from the variable to the invariable, but as a network of correlations between the two levels of signifying reality.
For a chemist natural signs are whatever can be seen, smelled and felt before, during and after an experiment. This assortment of signs is actively linked to that other group of signs devised by chemists to communicate among themselves - the 'language of chemistry'. This latter group also comes into play before, during and after an experiment. Ursula Klein has visualized the dynamic interpenetration of these two systems in Dumas' laboratory in the 1830s. While striving to 'read' the changes taking place in the flask during the chlorination of alcohol, Dumas was simultaneously manipulating 'paper-tools' in such a way that his "formulas became a surrogate for the concrete measurement of the quantities of substances involved in chemical transformation" (Klein 1997, pp. 33-34).
The accelerating trend toward designating compounds by symbols paralleled the growing complexity of the compounds to which they referred. Ironically, late 19th century initiatives to reform the nomenclature tended to reinforce this trend, because the new systematic names tended to obscure important functional relationships and be unwieldy as well (Verkade 1985, pp. 51-52). By far the greatest impetus for the move to symbolic representation was the creation, in the 1860s, of the structural theory of organic compounds (this crucial and extensively studied development is well summarized in Brock 1993, chaps. 6 and 7). Dependent on the earlier standardization of atomic weights and the development of the valency concept, structural theory held that atomic position as much as atomic character determines molecular properties. Creation of this theory would have been impossible without the use of symbolic notation - no nomenclature then available could have embodied the essential concepts. Furthermore, given the continuing association of alphabetic characters with ponderable combining weights, exploration of relational possibilities at the microscopic level might well have been inhibited by use of those symbols.
That the internal arrangement of atoms within a molecule was a major determinant of chemical behavior had become clear by the mid-19th century. Whether such arrangements were accessible to chemists and amenable to representation was, however, a matter of active and often heated debate. The implicit pull toward a structural interpretation of non-linear formulas is made evident by the frequent cautions against such interpretations: "It need hardly be mentioned that such [rational] formulae are not intended to express how the atoms are arranged in space, because of this we are totally ignorant." (Schorlemmer 1874, p. 32) In the very year these words appeared van't Hoff published a short paper in which a knowledge of atomic positions and the consequences thereof were forthrightly asserted (Brock 1993, pp. 259-63).
In this climate of assertion, denial, doubt and agnosticism, Kekulé's hexagon - the first chemical symbol of lasting consequence totally free of alphabetic characters - made its first appearance. The protean nature of this sign is shown by the variety of meanings attached to it by Kekulé and his successors.[5] The bare hexagon that made its appearance in 1865 (Figure 7a) did not specify the position of the benzene carbons, as had been universally assumed. Rather, it signified the equivalence of the six hydrogen atoms. "Indeed, Kekulé's intent seems to have been abstract and schematic, not really structural." It was Adolf Claus who in 1866 gave the hexagon its familiar signification, apparently without objection from Kekulé (Rocke 1985, pp. 371-72). What then did/does this figure mean to those who read it?
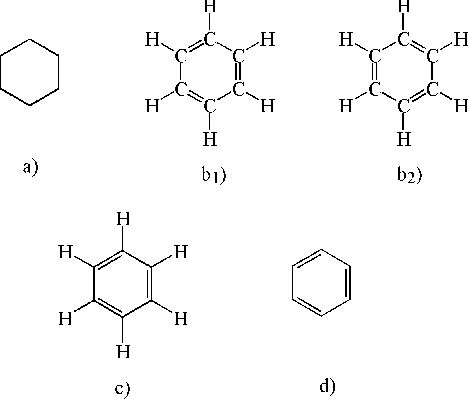
Figure 7. Various structural representations of benzene.
Recall that the chemical hexagon made its appearance just when the meaning and function of pictorial representation in general was in a state of great flux. The optical verisimilitude of photographs seemed to herald the perfection of the Renaissance 'window', an ideal representation whose transparency allowed the viewer direct access to the reality behind it. Before photography, painters had been the purveyors of optical mimesis; the advent of photographs provoked among younger painters a counter emphasis on the constructed nature of paintings. Within this frame of reference the question may be restated as follows: is the hexagon to be understood as a photograph or as a painting?
The case for the 'photograph' was put by Alexander Mikhailovich Butlerov, who claimed that
there will be possible only ONE such rational formula for each substance. If then the general laws will have been derived which govern the dependence of the chemical characteristics of the substances on their structure, such a formula will express all these characteristics Time and experience will teach us best how the new formulas will have to appear if they are to express chemical structure. (Butlerov [1861], p. 291)
Butlerov was reviving the dream of Condillac and in a sense even of the alchemists, of a signifier that would express the essence of the signified (Crosland 1996, pp. 226, 238). Kekulé himself seems to have been uncertain of what status to accord his benzene symbol and/or what he dared assert publicly. Although "proceeding with caution," notes Rocke, "Kekulé seems never to have seriously doubted the cyclohexatriene structure once he had it". Yet he drew the cyclohexatriene structure only once or twice when he first published his theory, and remained thereafter "curiously shy about using any resolved structural formulae for aromatics" (Rocke 1985, pp. 371-379). Furthermore, Kekulé was apparently content to allow the misinterpretation of the hexagon to stand - which brings us to the beguiling business of the disappearing double bonds.
In contemporary usage Figure 7b1 or 7b2 would be the most detailed structural formula for cyclohexatriene, 7c and 7d progressively less so but equally acceptable. In J. B. Cohen's 1923 survey of structural organic chemistry assorted typographical versions of 7a-7d, as well as other variants, are scattered throughout the text without apparent relation to context. There seem to be some general patterns with respect to different usages, but they are not invariable. It thus seems plausible that the chameleon-like quality of these symbols is precisely what accounted for their success. They could be tailored to different interests - synthetic chemists interested principally in isomeric distinctions could dispense with the double bonds, while their more theoretically-minded brethren could put them in; a range of theoretical commitments could be accommodated. There is a certain irony in the fact that while chemists were increasingly adopting a realist stand vis-à-vis molecular structure they could not avoid representations of those structures that were inherently ambiguous.[6] Yet those representations were to become increasingly indispensable, in part for reasons touched upon by Kekulé himself.
Opining that anyone who understood structure theory could have arrived at the benzene theory, Kekulé attributed his success to his "more eclectic approach to theoretical schools, and his 'irresistible need for graphic imagery [Anschaulichkeit]'" (Rocke 1985, pp. 366-377). The second of these attributes favored not only Kekulé's priority in arriving at the benzene hypothesis but its very rapid acceptance as well, because such a graphical formulation would fit very 'naturally' into the visual culture of chemistry.
In fact, once molecular realism had become received wisdom in the chemical community (by the end of World War I) the manipulation of graphical formulas became more and more a part of 'normal' chemistry. Textbooks devoted a decreasing proportion of space to the description of actual isolations and syntheses and correspondingly more to reaction equations (which, significantly, are often referred to simply as 'reactions'). In a very real sense, the molecular formulas, arranged in equations according to appropriate syntactical rules, allowed chemists to 'see' what was happening in reaction vessels otherwise devoid of visual clues. With the advent of computational chemistry we are even able to perform 'experiments' on these 'structures'.[7]
The system of chemical structures can represent far more than one or another class of compounds; its generative powers are limitless (although not unconstrained - but that is true of natural languages as well). Mestrallet undertook to seek out the source of this generative power, and it was her great insight to locate it in certain structural similarities between chemical formulas and equations, on the one hand, and words and sentences on the other. The 'languages' that are constituted by these two sets of building blocks show little superficial resemblance, yet they share in part a crucial underlying feature, their "double articulation" (Mestrallet 1981, pp. 23-25; Mounin 1981, p. 220).
The meaning of a sentence usually goes beyond the sum of meanings contained in its constituent words (the basic semantic units of language), and the creation of meaning intensifies as one proceeds to more complex linguistic entities. By contrast, the decomposition of words yields only non-signifying units (letters and clusters thereof). 'Meaning' cannot therefore be found in the ultimate constituents of language but only in their permutation.
The system of chemical representation is based analogously on a small repertoire of symbols, capable of a remarkable number of permutations. There are only two fundamental types of symbols, those representing atoms and those representing bonds. The most consequential distinction between them and the building blocks of linguistic systems is that chemical symbols are not completely devoid of signification. Like words, however, chemical formulas are enormously dependent upon context to crystallize their meanings. Only the context can tell us if the formula H2O represents a molecule or a mole; a gas or a liquid; an acid or a base; a nucleophile or electrophile.
The resemblance of structures to words has its limits, not least because structures are not conventional signs but rather iconic ones. A conventional signifier such as 'dog' has no necessary connection with the signified concept dog, and few of us scanning the signifier dwell on the letters 'd', 'o', and 'g'. 'Dog' is transparent - we see through it directly to dog. By contrast, to the extent that chemical symbols are iconic and meant to resemble that which they represent, the symbols themselves become the objects of scrutiny and lose their transparency. The benzene case provides yet further examples.
If all the valences in the benzene formula are explicitly accounted for, then either 7b1 or 7b2 would be perfectly valid and perfectly equivalent classical equations. However, if the 'true' structure of benzene is taken to be either of these then there are apparent discrepancies between the behavior 'predicted' by the representation and that found empirically. (Note that no such problem arises with the signifier 'benzene' - it 'predicts' nothing.) For Kekulé, one way out of this quandary was to postulate a rapid, ceaseless interconversion of 7b1 and 7b2; thus both were 'true' structures of benzene. For many chemists, discomfort with either the discrepancies and/or Kekulé's resolution of them resulted in a preference for the bare hexagon.
In the early 1930s Linus Pauling inverted Kekulé's solution and declared that neither 7b1 nor 7b2 represented benzene (Pauling 1940, pp. 128-130). According to Pauling these so-called 'resonance structures' actually referred to trial wavefunctions that could be combined mathematically to approximate the 'true' wave function.[8] Pauling (1940, p. 11) allowed that "there is an element of arbitrariness in the use of the concept of resonance". Some of us who teach chemistry to undergraduates are less circumspect and label resonance structures 'fictions'. In one commonly used simile 'real' benzene is likened to a rhinoceros, a real animal that can be allegedly conceived of as a hybrid of two fictional animals, the unicorn and the dragon. Regardless of the simile's pedagogical value, its use highlights the blurring of boundaries between the real and fictional and the role of the latter in chemical thought.
The ability to evoke a fictional world indistinguishable from the real one is another characteristic that the chemical code shares with natural languages. That ability has been long recognized and not always applauded; wrote one author of an elementary textbook (Freer 1895, pp. iii-iv): "Chemical equations I have avoided as much as possible [their] too frequent use may lead to the view that all reactions which can be so formulated must in reality take place." This danger must be balanced against the fact that "molecular representations are tools for modifying reality " (Mestrallet 1981, p. 21).
In all fields of science hypothesizing about the as yet unknown has been a spur to action. In physics and (until recently) biology that has meant searching for the hypothesized unknown. The response of chemists is an attempt at creating it. Chemists are imbued early on with the challenge of the hypothetical. At WPI some years ago our first semester sophomore text was entitled Nonexistent Compounds (Dasent 1965). Literally speaking, this was a work of science fiction. In common with many other varieties of fiction this genre used the imaginary to illuminate the real, and it often provokes its readers into turning 'fiction' into 'fact'.
The dynamic relationship between chemists and their language has been succinctly captured by Hoffmann and Laszlo (1991, pp. 3, 14): "The writing of a structure is not innocent Knowing the 'name' of a compound, which means its structure, gives the chemist tremendous power over the molecule. A range of its properties, its behavior are implied by that structure." I concur with this claim and would further assert that when we choose to employ one out of the gamut of available representations for a molecule, then in a sense we give that sign power over us. Certain possibilities appear 'before our eyes'; others are veiled from view.
Mestrallet classifies structural formulas as "non-verbal systems of communications, composed of novel and unique signs for a novel and unique reality". As her work continually stresses, communication is as much the raison d'être for the chemical code as representation: "Our systems have undergone an evolution parallel to that of languages because the conditions necessary for communication in chemistry are similar to those of languages" (Mestrallet 1981, pp. 29, 31). Structural formulas should therefore be thought of as 'paintings', constructions in which some aspects of molecular reality are suppressed, others highlighted (Hoffmann and Laszlo 1991, pp. 4-5; Mestrallet 1981, p. 10). Butlerov's dream of a single molecular structure is unrealizable both because of the dynamic nature of molecules and the needs of communication. Indeed, it is not clear how these two factors are to be disentangled.
If we think of chemical signs as nothing more than 'fingers' pointing to a predetermined reality, we slight their unique and invaluable creative functions. Substances and their signs come into being together, and their evolving symbiosis accounts in no small part for the power and potential of each.
* This paper expands and updates a talk given at the annual conference of the Society for Literature and Science, 25 September 1989. It has its origins in the author's participation in a 1978 NEH Summer Seminar, "The Functions of Discourse in Literature and Science", Michigan State University, directed by E. Fred Carlisle.
Anderson, W.: 1984, Between the Library and the Laboratory, Johns Hopkins University Press, Baltimore.
Anderson, W.: 1985, 'Rhetoric and Nomenclature in Lavoisier's Chemical Language', Topoi, 4, 165-169.
Biot, J.-B.: 1854, 'Introduction' to: Auguste Laurent, Méthode de Chimie, Mallet-Bachelier, Paris.
Brock, W.H.: 1993, The Norton History of Chemistry, W.W. Norton, New York.
Brown, H.C.: 1977, The Nonclassical Ion Problem, comm. Paul von R. Schleyer, Plenum, New York.
Brown, L.A.: 1994, The Story of Maps, Bonanza Books, New York.
Bunn, J.H.: 1981, The Dimensionality of Signs, Tools, and Models, Indiana University Press, Bloomington.
Butlerov, A.M.: 1971, 'On the Chemical Structure of Substances' [1861], Journal of Chemical Education, 48, 289-291.
Carlisle, E.F.: 1980, 'Literature, Science, and Language: A Study of Similarity and Difference', Pretext, 1, 39-72.
Cohen, J.B.: 1923, Organic Chemistry for Advanced Students. Part II. Structure, 4th ed., Longmans Green, New York.
Crosland, M.P.: 1962, Historical Studies in the Language of Chemistry, Harvard University Press, Cambridge.
Crosland, M.P.: 1996, 'Changes in Chemical Concepts and Language in the Seventeenth Century', Science in Context, 9, 225-240.
Dagognet, Fr.: 1969, Tableaux et langages de la chimie, Editions du Seuil, Paris.
Dasent, W.E.: 1965, Nonexistent Compounds: Compounds of Low Stability, Marcel Dekker, New York.
Freer, P.C.: 1895, The Elements of Chemistry, Allyn and Bacon, Boston.
Geoffroy, E.-Fr: 1996, 'Table of the Different Relations Observed in Chemistry between Different Substances - 27 August 1718' [1718], Science in Context, 9, 313-320.
Gregory, W.: 1860, Handbook of Organic Chemistry; For the Use of Students, A. S. Barnes and Burr, New York.
Greimas, A.J.: 1987, 'Toward a Semiotics of the Natural World', in: On Meaning: Selected Writings in Semiotic Theory, trans. P.J. Perron and F.H. Collins, University of Minnesota Press, Minneapolis, pp. 17-47.
Guédon, J.-Cl.: 1980, 'Le statut épistémologique de la réaction chimique de l'encyclopédie à Gay-Lussac', Colloque Gay-Lussac, Ecole Polytechnique, Paris, pp. 103-131.
Guiraud, P.: 1975, Semiology, trans. G. Gross, Routledge and Kegan Paul, London.
Guyton de Morveau, Baron L.-B.; Lavoisier, A.-L.; Berthollet, C.L.; de Fourcroy, A.F.: 1787, Méthode de nomenclature chymique, Cuchet, Paris (translations are from Anderson 1984).
Hoffmann, R.; Laszlo, P.: 1991, 'Representation in Chemistry', Angewandte Chemie International Edition in English, 30, 1-16.
Hoffmann, R.: 1995, The Same and Not the Same, Columbia University Press, New York.
Holmes, F.L.: 1989, Eighteenth-Century Chemistry as an Investigative Enterprise, Office for History of Science and Technology, University of California at Berkeley, Berkeley.
Holmes, F.L.: 1996, 'The Communal Context for Etienne-François Geoffroy's Table des rapports', Science in Context, 9, 289-311.
Jordanova, L.: 1986, Languages of Nature: Critical Essays on Science and Literature, Rutgers University Press, New Brunswick.
Kim, M.G.: 1992, 'The Layers of Chemical Language, I: Constitution of Bodies v. Structure of Matter', History of Science, 30, 69-96.
Klein, U.: 1994, 'Origin of the Concept of Chemical Compound', Science in Context, 7, 162-204.
Klein, U.: 1996, 'The Chemical Workshop Tradition and the Experimental Practice: Discontinuities within Continuities', Science in Context, 9, 251-287.
Klein, U.: 1997, 'Paper-Tools and Techniques of Modelling in Nineteenth-Century Chemistry', in: Nineteenth-Century Chemistry: Its Experiments, Paper-Tools, and Epistemological Characteristics, Preprint 56, Max-Planck-Institut für Wissenschaftsgeschichte, Berlin, pp. 27-47.
Laszlo, P.: 1993, La Parole des Choses, ou, Le Langage de la Chimie, Hermann, Paris.
Lavoisier, A.L.: 1790, Elements of Chemistry: In a New Systematic Order Containing All the Modern Discoveries, trans. R. Kerr, William Creech, Edinburgh.
Meier, G.F. [1718-1777], quoted by Ch. Rosen, 'The Great Inventor', New York Review of Books, October 9, 1997, 51-55.
Mestrallet Guerre, R.: 1980, Communication, Linguistique et Sémiologie. Contribution à l'étude de la sémiologie. Études sémiologique des systèmes de signes de la chimie, (Thesis), Faculty of Letters, Universitad Autònoma de Barcelona.
Mestrallet Guerre, R.: 1981, Communication, Linguistique et Sémiologie. Contribution à l'étude de la sémiologie. Études sémiologique des systèmes de signes de la chimie, Bellaterra, Barcelona (extended abstract of Mestrallet 1980, available through the Biblioteca Nacional, Madrid; a briefer summary is provided by Mounin 1981).
Miller, W.A.: 1869, Elements of Chemistry: Theoretical and Practical, 4th ed., Part III: 'Organic Chemistry', Longmans, Green, Reader, and Dyer, London.
Mounin, G.: 1981, 'A Semiology of the Sign System Chemistry', Diogenes, Spring/Summer, 216-227.
Nye, M.J.: 1993, From Chemical Philosophy to Theoretical Chemistry: Dynamics of Matter and Dynamics of Disciplines, 1800-1950, University of California Press, Berkeley.
Paradis, J.: 1983, 'Bacon, Linnaeus, and Lavoisier: Early Language Reform in the Sciences', in: P. Anderson et al. (eds.), New Essays in Technical and Scientific Communication: Research, Theory, Practice, Baywood Press, Farmingdale/NY, pp. 200-224.
Pauling, L.: 1940, The Nature of the Chemical Bond, and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry, 2nd ed., Cornell University Press, Ithaca.
Priesner, C.: 1989, 'How the Language of Chemistry Developed', Chemistry International, 11, 216-238.
Roberts, L.: 1991, 'Setting the Table: The Disciplinary Development of Eighteenth-Century Chemistry as Read Through the Changing Structure of Its Tables', in: P. Dear (ed.), The Literary Structure of Scientific Argument: Historical Studies, University of Pennsylvania Press, Philadelphia.
Rocke, A.J.: 1985, 'Hypothesis and Experiment in the Early Development of Kekulé's Benzene Theory', Annals of Science, 42, 355-381.
Schorlemmer, C.: 1874, A Manual of the Chemistry of Carbon Compounds; Or, Organic Chemistry, Macmillan, London.
Schuster, J.A.; Yeo, R.R.: 1986, The Politics and Rhetoric of Scientific Method: Historical Studies, Reidel, Dordrecht.
Siegfried, R.: 1982, 'Lavoisier's Table of Simple Substances: Its Origin and Interpretation', Ambix, 29, 29-48.
Tongchai Winichakul: 1994, Siam Mapped: a History of the Geo-Body of a Nation, Silkworm Books, Chiang Mai/Thailand.
Verkade, P.E.: 1985, A History of the Nomenclature of Organic Chemistry, Reidel, Dordrecht.
Wightman, W.P.D.: 1963, 'Essay Review: The Language of Chemistry', Annals of Science, 19, 259-267.
Copyright Ó1998 by HYLE and Stephen J. Weininger