http://www.hyle.org
Copyright © 2007 by HYLE and Klaus Ruthenberg
HYLE Biography |
František Wald (1861-1930)by Klaus Ruthenberg*František (Franz) Wald was born on January 9, 1861, in Brandysek, Bohemia (Austria). His father had been an immigrant from Chemnitz, Saxony (Germany), his mother originated from Neudeck, North West Bohemia. Because of the Prussian War in 1866 the family moved to Kladno near Prague where Wald went to Czech schools. After having passed the German Grammar School at Prague he studied technical chemistry at the German Technical University Prague. He passed the first state examination in 1881 and left one year later without final examination which was not required for an industrial appointment. In 1882 he was appointed to the Prazska Zelezarska Spolecnost Ironworks in his hometown Kladno. One year later Wald married Josefa Radkovska with whom he had five children. In 1886 he was appointed chief chemist of that company where he stayed until 1908, when shortly after the death of his beloved wife he accepted a call (from 1907) to the Czech Technical University, which was called Prague Polytechnic at the time and which had recently been separated from its German counterpart. The chair for "theoretical chemistry, physical chemistry and metallurgy" was built ad personam: Eminent personalities of scientific and political life like Tomas Masaryk (philosopher and later president of the first Czechoslovakian Republic), Ernst Mach (physicist and philosopher), and Wilhelm Ostwald (chemist and philosopher) had fostered the academic career of František Wald at least since the turn of the century. During his university career he served twice as a dean of the chemistry department (1909/10 and 1915/16) and as rector of the university in 1919/20, the crucial year of university reorganization after the disintegration of Austria. The new name of the institution since then has been Ceske Vysoke Uceni Technicke (CVUT), but the chemistry institutes were separated from the other departments in 1952. In 1928 he suffered from an apoplectic fit and retired from lecturing, and shortly after his formal retirement he died two years later. For further biographical information in English see Druce 1944 and Pinkava 1987. The published works of František Wald are mainly dedicated to general and fundamental chemical topics (see the bibliography in Simek 1931). Only few original works exist on analytical problems (e.g. the analysis of manganese in steel), which were published before he moved to academia. As to the realm of chemical (mostly stoichiometric) theories there are about 70 papers and two monographs. The frequency of his publications greatly varies over time: from 1881 to 1905 almost two thirds were already printed; the main published work after his call to the university is his second book Chemie Fasi (‘Chemistry of Phases’) from 1918. This monograph was published in Czech only. A reprint with a German translation by Wald’s son František jr. and E. Klima was prepared in 1929, but not published, supposedly due to the death of the author, before 2004 (Ruthenberg 2007). The selection of journals for his original contributions, as well as his choice of language, is intriguing. The first series of 12 papers written in German were published in Zeitschrift für Physikalische Chemie (1887-1900), the second series of 7 papers in Annalen der Naturphilosophie (1900-1909). Both periodicals had been founded and were mainly run by Ostwald. Only few articles – most of which repeated earlier subject matters – can be found elsewhere afterwards (e.g. in Chemiker-Zeitung). Since he was a Bohemian patriot, Wald published also in Czech: 17 papers (1888-1916) appeared in Chemicke Listy, a journal founded in 1877 to foster national chemistry in Bohemia. Wald’s efforts in theoretical chemistry can be divided into three historical periods. As a young industrial scientist who was busy in analytical chemistry during the day, he managed to work and write on thermodynamics in his spare time, which resulted in his first book Die Energie und ihre Entwertung from 1889 and in some journal papers in the first period up to 1893. In this monograph he expressed his anti-atomistic view: "As far as I am concerned the roots of this law [the second law of thermodynamics] go much deeper [than any molecular hypothesis can tell], and to correlate molecular hypothesis and entropy is rather a lucky achievement for this hypothesis than advantageous for the second law of thermodynamics" (p. 104, my translation). Wald has been an anti-atomist from the very beginning of his scientific work. He did not categorically deny the possibility of a future success of the atomistic hypothesis, but "in the meanwhile this hypothesis should remain only a makeshift for our weak power of comprehension; by no means should it be used to replace the causal law that is expressed in the theorem of the devaluation of energy" (p. 105). Because of his anti-metaphysical and phenomenalist point of view Wald was warmly received by Ernst Mach (1838-1916). In his Principien der Wärmelehre from 1896, Mach complied with Wald’s views, cited his "roots-statement" (p. 364), and benevolently mentioned his anti-atomistic efforts in chemistry (p. 360). Indeed, it is very likely that Wald had been influenced by Mach’s phenomenalism in the early phase of his development. Between 1867 and 1895 Mach was professor at Prague, and the student Wald could have attended his lectures easily. Moreover the correspondence between the two scholars which reached at least until 1910 (Pinkava 1987) indicates their friendly relationship. As to his relation to energetism, however, the above-mentioned quote shows that Wald, like Mach, did not think of energy as the concept of highest priority in science. In the conclusion of Chemie Fasi, he said: "Around 1893 I started to scrutinize the whole chemical thinking in order to refine it from hypothetical imagination, and to bring forward only chemical experience in phenomenological description after the method of Mach" (p. 223, my translation). Hence, he stood in the tradition of Mach’s sensualistic epistemology rather than in that of Ostwald’s energeticist scientism even before 1893, although he benevolently mentioned Ostwald’s point of view particularly in some of his early writings. However, from that year on, Wald tried to show (first in Czech) that the stoichiometric laws can deductively be derived from a priori principles. He argued that the concept of chemical elements already implies the laws of simple and multiple proportions and that these laws were no empirical generalizations. His argumentation drew on the concept of phase and the Gibbsean phase rule. The latter describes the relation between the composition of a system of substances, its number of phases, and the state of the system, which is expressed by the number of the degrees of freedom. Wald developed systems of algebraic equations to represent the conservation of constituents in a chemical reaction: x1a1 + x2a2 + x3a3 + … + xkak = 0 x1b1 + x2b2 + x2b3 + … + xkbk = 0 … x1s1 + x2s2 + x2s2 + … + xksk = 0. There are k substances reacting, xk are the mass units of substances 1, 2,…, k. If this system of equations is developed into a determinant and if the elements of every column are multiplied by factor ys, which Wald called ‘valency’, the following system of equations is obtained: yaa1 + ybb1 + ycc1 + … + yss1 = 0 yaa2 + ybb2 + ycc2 + … + yss2 = 0 … yaak + ybbk + ycck + … + yssk = 0 For instance, if we take the thermal decomposition of calcium carbonate CaCO3 →
CaO + CO2
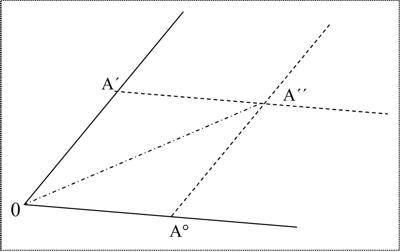
the conclusion will be correct: yCa + yC + 3yO – yCa + yO – yC – 2yO = 0 However, if we try to apply this approach to chemical reactions with altering oxidation numbers, we fail. For instance, for the Boudouard equilibrium (C + CO2 → 2 CO), we receive: yC + 2yO + yC – yC – yO ≠ 0. In fact, as Pinkava has already stated, these equation systems "… are evidently the mathematical expressions of the number of atoms and electroneutrality of compounds" (Pinkava 1987, p. 18), rather than algorithms to calculate valence numbers for any given chemical process. Wald’s first attempts to establish a phenomenalistic, anti-atomistic theory of chemistry exhibited drawbacks, most of which he realized himself very soon. Although his claims about the deductive nature of the stoichiometric laws and his inquiries on the substance concept were impressive and new, he had not yet found an appropriate mathematical approach. He received critical and even disapproving responses from colleagues in that period. Accordingly, as can be inferred from his correspondence, these years of his strive for the new theory took much of his drive. The third period of Wald’s theoretical work was still dedicated to fundamental stoichiometry. However, he skipped the phase rule as a starting point as well as the algebraic approach. That appeared necessary to him when in the early the 20th century he realized that the concepts of ‘pure substance’ or ‘pure constituent’, which were prerequisites in the phase rule, did presuppose what could only be, if any, the result of his theoretical efforts. If there are pure substances, these could be found only by the correct operations, because the substances we call elements in chemistry do not pre-exist. Rather, these substances are preparations, but the proper ways of preparation are neglected by the usual (atomistic) chemists. In what is presumably the last letter to Mach from May 1910 he wrote: "At the moment I am sitting at the sketch board the whole day construing poly-dimensional figures of chemical phenomena" (Pinkava 1987, p. 135, my translation). In his book Chemie Fasi he started with one-dimensional graphic representations of what he called the fundamental relations of mass-independent chemical qualities (density, color, conductivity, etc.) of phases. Wald gave three separate definitions of the concept of phase. Firstly a phase is described as "… any part in a mixture in equilibrium that is recognized by the senses and is a homogeneous part, differentiable from the others" (p. 112, my translation; emphases original). Secondly: "Substances [Stoffe] which, by coming into contact, can form one single substance [Stoff] without any spatial discontinuity arising during such a process belong to one and the same phase" (p. 133, my translation). And finally: "In the concept of phase we put together the constant mixture of similar products which apparently follow a state equation of their own" (p. 135, my translation). The qualities of phases are the sum of specific properties, and should be considered immeasurable according to Wald. In Figure 1 (from Wald 2004, p. 137), the point A’’ denotes the resulting sum of chemical qualities of two mixed guide-links (Leitglieder) of the same phase A, the intensity of which are given by the coordinates A° and A’. For example, for the phase A these two guide-links A° and A’ could be the variables for more or less pure water on the one hand and a saturated common salt solution on the other, and A’’ the representation of a mixture of these. If the relation of the intensities is kept equal, the result will be the same linear, again one-dimensional sum of qualities which is represented by the line 0–A’’.
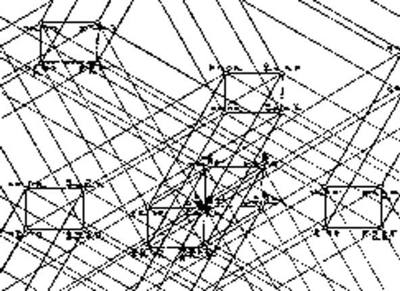
Figure 1: The start-up of a geometrical representation of chemical processes. If the mixing of two Leitglieder does not yield a change of any quality we count as significant then these two Leitglieder are of the same chemical composition. Wald claimed that, starting from substances which are undefined regarding their chemical composition, pure substances could be obtained by a finite number of such mixing procedures. In Chemie Fasi, he ended up with extremely complicated reaction networks (Figure 2) which he unfortunately did not clarify by examples. A full reconstruction of his final theory is still missing.
Figure 2: Part of a reaction-net or simplex (Wald 2004, p. 184). František Wald was a lonely man, at least in terms of his scientific endeavors. During the first and second period of his theoretical efforts he still could hope to become a fully accepted member of the scientific community. He published in high-rated journals, received promotion from famous and influential scientists, and finally was appointed professor at a university. Nevertheless, when he began his university career in 1908, Wald was already a scientific loner. The acceptance of his papers by the Zeitschrift für Physikalische Chemie ended in 1900 because Ostwald forced him to change to the new Annalen der Naturphilosophie (Pinkava 1987, pp. 104-106). And the row of his articles reluctantly submitted to the latter journal ended in 1909. However, just during these years Ostwald repeatedly had asked him for a book contribution, which Wald never wrote. The death of his wife and his patriotic activities during the funding of the Czechoslovakian Republic may also have been influential on his exit from normal chemical science. However, there were also other factors related to the core of Wald’s work as for example the following. Around 1904, a prominent former anti-atomistic comrade took up the inspiration and the initial ideas of Wald’s second period to produce a book that was explicitly meant to be (and actually still is) the textbook of anti-atomistic chemistry. In his Prinzipien der Chemie from 1907 (The Fundamental Principles of Chemistry 1909), Ostwald was fair enough to mention Wald in his preface, but in the course of his text he did not refer to a single piece of Wald’s publications. Both scientists seem to have had no written correspondence about the Prinzipien whereas their published exchange of letters ends only in 1913. Although Ostwald did not really copy the thoughts of the Bohemian, that book must have been like a shock for František Wald. Although he certainly did not agree with some of Ostwald’s claims in the Prinzipien, e.g. the explicit energetical point of view, the inconsistencies, and some awkward definitions, he must have realized almost immediately that this book had something impressive to it. Indeed it was a frontal attack on the entire realm of atomistic chemistry, the realm which Wald could have considered his property to a considerable extent for quite a long time. Moreover that attack was ridden by the man who on the one hand had promoted him to achieve an academic position, and who on the other increasingly shoved aside his manuscripts. The main question at this situation might have been: What is left? Together with his already solitary status in academic chemistry this must have been a heavy burden for the Bohemian scholar. Already one year before the release of
Ostwald’s Prinzipien,
in May 1906, Wald wrote to Mach (Pinkava 1987, p. 122, my translation),
expressing his increasingly depressive mood: "It is my destiny that I
staked everything on one card…" AcknowledgementsI like to thank Jiři Wald for the generous
gift of a
copy of the archived ‘family’ files of Professor František Wald and Dr.
Eva Bohacova for the kind and useful help with the archives of the
CVUT. Cordial thanks go also to Prof. Dr. Jaap van Brakel, Institute of
Philosophy, University of Leuven, who catalyzed my refreshed interest
in phenomenalist chemistry. Selected works by WaldWald, F.: 1889, Die Energie und ihre Entwertung, Wilhelm Engelmann, Leipzig. Wald, F.: 2004, Chemie Fasi, Univerzita Karlova v Praze, Prague (reprint of the original from 1918/1929 with German translation). Wald, F.: 1896, ‘Chemistry and its Laws’, Journal of Physical Chemistry, 1, 21-33. Wald, F.: 1931, ‘Foundations of a Theory of
Chemical
Operations’, Collection des travaux chimiques de Tchecoslovaquie,
3, 32-48. ReferencesDruce, G.: 1944, Two Czech Chemists – Bohuslav Brauner (1855-1935) František Wald (1861-1930), The New Europe Publishing Company Ltd., London. Mach, E.: 1900, Die Principien der Wärmelehre, 2nd ed., Johann Ambrosius Barth, Leipzig. Ostwald, W.: 1909, The Fundamental Principles of Chemistry, Longmans, Green, and Co., New York etc. Pinkava, J. (Ed.): 1987, The Correspondence of the Czech Chemist František Wald with W. Ostwald, E. Mach, P. Duhem, J.W. Gibbs and other Scientists of that Time, Rozpravy Ceskoslovenske Akademie Ved, Prague. Ruthenberg, K.: 2007, ‘Saving Chemical Phenomena outside the Scientific Community’ (Review of Wald: Chemie Fasi), Foundations of Chemistry, in press. Šimek, A.: 1931, ‘Bibliography of scientific communications published by František Wald’, Collection des travaux chimiques de Tchecoslovaquie, 3, 5-8. Klaus Ruthenberg: |