http://www.hyle.org
Copyright © 2015 by HYLE and OCHIAI Hirofumi
Philosophical Foundations of StereochemistryOCHIAI Hirofumi*
1. IntroductionStereochemistry, which is a sub-discipline of chemistry, is concerned with molecules as entities with shape and structure. This fact itself poses questions about the legitimacy of this science because neither shape nor structure is taken any longer as a solid scaffold on which to construct philosophical as well as scientific arguments, as is described in the following. Stereochemistry involves studies of isomers and stereo-chemical reactions. The former focus on the static aspect of molecules, and are concerned with the topological as well as the geometrical arrangement of atoms in molecules (as is exemplified by n-propanol and iso-propanol, and cis- and trans-butenes, for instance). They are also concerned with the spatial disposition of atoms in molecules (as is exemplified by enantiomers, one enantiomer being the mirror image of the other). Enantiomorphy (incongruence with one’s mirror image) raises philosophical issues concerning the relationship between chemistry and the nature of space (Le Poidevin 2000). The latter, i.e., the study of stereochemical reactions, focuses on the control of stereo-specific and/or stereo-selective reactions. The way the trajectory of reacting molecules is controlled to generate one isomer over another has been described with reference to the shape and structure of molecules. That kind of study is concerned with the dynamic aspect of molecules. As is suggested by this argument, stereochemistry stands on the model, which envisages molecules as composed of atoms that are linked together by chemical bonds. In other words, the molecule is taken as an entity that has a definite shape and structure, and behaves like mechanical objects in the world of possible experience. Taking into account the fact that submicroscopic entities like atoms and molecules are under the control of quantum mechanical principles, this model seems to stand on a prima facie unacceptable premise, and hence needs careful investigation. We call it the classical model of the molecule in this paper because it is based on the classical concept of the molecule, and also because it is in marked contrast to the quantum mechanical view of the molecule (Ochiai 2013). The point to be noticed is that it does not seem to be a likely model to represent the dynamic aspect of the molecule because of its character described above.[1] Actually, we know that it is empirically adequate in the analysis of stereo-chemical reactions, and find it useful for designing molecules and molecular transformations. It is obvious from this fact alone that the classical model needs thorough investigation from a philosophical as well as a scientific point of view. The truth is that until recently we did not have precise knowledge about the physical shape of molecules in dynamic motion nor about the relationship to the structural problems. According to the above discussion, four questions are addressed in this paper, and summarized as follows: The first question is whether it is allowed to treat molecular events, which are subject to quantum mechanical principles, by way of the classical model. The discussion rests on the claim that the notion of structure is useful in a many-body system with strong Coulombic interaction. This will be discussed in Section 2 along with the second question. The second question is whether (or in what respects) it is allowed to treat dynamic processes of stereochemical transformation by way of the classical model. Also discussed in Section 2 are the history of stereochemistry and the features of modeling practice in chemistry. The third question, discussed in Section 4, is whether or not the classical model can be proved true by physical measurements of molecules. It concerns the relationship between shape and structure, the former being the product of physical analysis, and the latter the product of modeling practice in stereochemistry. The fourth question is concerned with enantiomorphism and its relationship with the nature of space; that is, the question discussed in Section 6 is whether or not enantiomorphism is explicable without recourse to the nature of space, or in other words, whether or not enantiomorphism has some implication for the philosophy of space. In the course of the discussion the relationship between models and the real system will be argued in Section 3, in which my philosophical view of scientific knowledge is presented. If the modal structure of the model is all we know about nature (or in other words, if the causal structure of the real system is beyond our reach), the choice of models must be of tremendous importance for our understanding of nature. In Section 5 the concept of the affordance will be introduced, together with some related concepts, which as a whole serve as a tool kit for the philosophy of chemistry. While the concept of affordance provides a philosophical basis for the choice of models, it may plunge into fallacious arguments without reference to the world outside chemistry. The argument in Section 6 about enantiomers, its relationship to the nature of space in particular, is worth consideration because it may present an obstacle to micro-reductionism (i.e., the view that chemical properties are wholly explicable in terms of intrinsic microphysical properties). 2. The classical model of the moleculeThe history of stereochemistry and the features of modeling practice which arose in the course of its development are relevant to topics discussed in this section. Unlike most chemical theories and concepts which can be taken as empirical generalizations of chemical phenomena, those of stereochemistry call for the kind of mathematical abstraction that van ’t Hoff drew on for his theory of the tetrahedral carbon atom. The idea of a bond that is rigid and not capable of changing directions could not be derived by simple inductive inference from chemical experience. In addition, a clear understanding of the relationship between chemical structure and the physical shape of the molecule had not been established before his day. The distance from empirical knowledge of compounds to the idea of stereoisomerism was beyond our imagination. In 1848 Pasteur showed, by optical resolution of sodium ammonium paratartrate, that the paratartrate salt consists of an equal number of left-handed and right-handed crystals. He tested each crystal for its effect on polarized light, and found the optical inactivity of paratartrate could be attributed to the equal number of left-handed and right-handed molecules, each of which cancelled the effect of the other. Pasteur was the first to show a distinct relationship between crystalline form, optical activity, and asymmetry at the molecular level (Ramberg 2003, pp. 33-35). The possible origin of asymmetry was, however, not pursued in terms of the structure of the molecule, and was left for others to explain. In 1869 Johannes Wislicenus, who had been immersed in the characterization of isomeric lactic acids, first suggested that the cause of what is now called optical isomerism could lie in a difference in the spatial arrangement of atoms. Although he was convinced that some sort of physical cause was necessary to explain the observed difference in the optical rotation of -lactic acid, Wislicenus could not make any concrete claims about it. It is worth noting that Wislicenus could write down the structures of - and -lactic acid by means of Crum Brown’s formula, an episode suggesting how hard it was to overcome the epistemological barrier between the chemical structure and the physical shape. This may be ascribed to the nineteenth-century understanding of the structural formula: it was taken as a symbolic sign representing the abstract concepts of chemical combination implied by valence (Ramberg 2003, pp. 50-52). Being symbolic and conventional but not an iconic image, the structural formula had no dimensionality at all. It was van ’t Hoff who gave physical reality to the structural formula, and transformed it from symbolic into iconic. In the course of explaining the isomerism of lactic acids van ’t Hoff tested arrangements of atoms by trial and error, and compared the number of possible theoretical isomers with the number of known isomers. In 1874 he came up with the idea that the cause of optical activity in a compound could be attributed to the presence of at least one asymmetric carbon atom in its structure. By assuming the tetrahedral arrangement of valences in each carbon atom, the optical activity of molecules could be inferred from structural formulas. For van ’t Hoff the tetrahedron was a graphic, literal representation of the arrangement of valences around the carbon atom, and in this respect it stood in marked contrast to the tetrahedron that Le Bel proposed as the molecular type. Le Bel drew on the French tradition of crystallography, and started from Pasteur’s discovery that optical activity was an indicator of asymmetry at the molecular level (Ramberg 2003, pp. 54-65). Thus, while the two models are similar in appearance, they were distinct in their modal structure. Compared with Le Bel’s, van ’t Hoff’s model was much easier to apply more widely. Large research programs were set up to test the hypothesis about asymmetric carbon atoms in addressing the stereoisomerism of unsaturated as well as saturated compounds. It should be noticed, however, that Le Bel’s model seemed equally promising in 1874 when a relatively low number of optically active compounds was known (Ramberg 2003, p. 65). Worthy of note in this story is that neither van ’t Hoff’s nor Le Bel’s model was of true-or-false character in a literal sense because they were not inevitable outcomes derived from chemical experiences. The way of modeling molecules might have been otherwise. Now we are investigating the features of the classical model of the molecule, which has been improved from the era of van ’t Hoff and Kekulé, and also from the electronic theory of organic chemistry. The first point to ask is in what respect and to what extent the classical model of the molecule is relevant to the reality of molecules which underlies observable chemical phenomena. The second point is concerned with questions raised in Section 1: Whether or not, or for what reason, the thus characterized model is allowed to delineate dynamic processes of molecular transformation? The idea that underlies the classical model of the molecule can be summarized as follows.
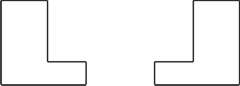
If a theoretical hypothesis is defined as a statement asserting some sort of relationship between a model and a designated real system, and if the relationship is of similarity (Giere 1988, p. 80), the hypothesis in stereochemistry will be that the molecule can be taken as an entity similar to a mechanical object in the world of possible experience. The implication of taking molecules as entities with definite shape and structure is huge because ‘molecular structure’ is ‘the essential concept that states the answer without knowing how to solve the macroscopic many-body problem’ (Woolley 1978). The contention is that it is of critical importance to differentiate ‘quantum structure’ from ‘classical molecular structure’. To state it in a more detailed way, since the configuration space used in quantum theory is an abstract Hilbert space, a molecule has no extension in space or time. In other words, ‘molecular structure makes no appearance in a quantum treatment of molecules starting from first principles’. In contrast, the quantum-mechanical description of molecules under usual experimental conditions has to be based on the time-dependent Schrödinger equation. It is realized by assuming molecular structure, on which we describe the situation in terms of a model time-independent Schrödinger equation for an individual molecule. We hold the nuclei at rest and calculate the electron distribution for the specified, fixed nuclear configuration. After this calculation was done, the modifications required because of nuclear motion are considered (the Born-Oppenheimer approximation). On the other hand the parity conservation requirement implies that an isolated molecule in a stationary state cannot exhibit optical activity because of the transitions between stationary states. This shows that molecular structure is a consequence of environmental perturbations rather than an intrinsic molecular property. In conclusion the molecular structure hypothesis of the Born-Oppenheimer approximation is rational (and so is the classical model of the molecule) to solve the macroscopic many-body problem because molecules in real systems are in interactions with environments. 3. Models and the real systemFor something to happen there must be something that makes it happen, whether or not it is observable to the naked eye. In other words, ‘observation – seeing with the naked eye – is not the test of existence’ (Cartwright 1983, p. 7). If observability is crucial for deciding whether or not the object exists, what science is possible for the experiments we do every day in chemical laboratories? Nothing can be known about what is going on in a stirred glittering solution just by looking at the reaction vessel. The empiricists’ naïve criterion is untenable in light of the accumulated achievements of modern science. The test of existence should be looked for in the causal power of the unobservable entity. When one can manipulate something unobservable so as to intervene in other things, using it as a tool for scientific investigation by exploiting its causal power, one cannot doubt its existence (Chakravartty 2007, p. 30).[2] As to the molecule there seems to be no room to doubt about its existence because, for instance, asymmetric syntheses of chiral substances have been successfully realized for years, and many of them are utilized as pharmaceuticals for their physiological activities. On the other hand, unawareness of the precise compositions and of the structure-activity relationship has sometimes caused serious problems for patients. One such unpleasant example is Thalidomide, which was a mixture of R- and S-isomer, and sold as a medicine to alleviate nausea and morning sickness in pregnant women. It was revealed afterward that adverse effects such as malformation of limbs in infants are caused by the S-isomer, while the desired medicinal effect is caused by the R-isomer only.[3] The causal power of these chemical substances demonstrates the existence of molecules and the effect of molecular structure as well. The question to ask about molecular structure is, however, not about being-or-not-being, but about the way it is. Can we know the truth of molecular structure in the literal sense of word? My answer is in the negative. The molecular structure we encounter in chemistry textbooks is a theoretical model with which to describe our chemical experience. It is an embodiment of theories through which abstract theories are made to correspond to the real system. The modal structure of the classical model of the molecule may or may not be relevant to the causal structure of the real system. It depends on the problem we are addressing. In order for a model to be recognized as adequate, not to say true, it is indispensable to show a causal connection between the modal structure of the model and the observable effect in a very special case; that is, by testing it in highly controlled experiments (Cartwright 1983, pp. 6-10). On the other hand, while Giere says that the modal structure is likely to have its counterpart in the real system in cases where such a connection is shown, I do not see it as enough to support realist belief (Giere 1988, p. 99). For instance, the force field theory presents us with an example against his claim. The chemical bond is not a minute spring connecting two rigid bodies, though some aspects of its work can be explained by comparing it to the mechanical force of a spring. Likewise, quantum chemistry, and the electronic theory of organic chemistry as well, focus on some specific aspect of bonding in the molecule. In fact, the bonding is nothing but an artifact of our thought. Thus, because there is no way for us to see the world as it is, we have to choose some aspect to focus on according to the aim of the investigation. Since no single model serves all purposes, we have to construct different models for different purposes. Therefore, asking ‘which is the right model?’ is a pertinent question only for a concrete setting, as Cartwright has pointed out. In other words, what is accessible to us is at most an empirically adequate model of the world. 4. Shape and structureTaking into account the fact that the way of modeling molecules is not fixed but depends on the viewpoint of investigation, as was exemplified by van ’t Hoff’s and Le Bel’s models, it will be fair to ask whether or not our model can be justified in light of something objective. The physical shape of the molecule may be taken as an adequate candidate, for shape has been regarded as a primary or essential quality of matter. Can the classical model of the molecule be justified by the physical analysis of molecules? To address this problem, however, we must first make clear what the physical shape of the molecule is. Shape is more than mere appearance, but less than intrinsic, because it takes time to be detected (Ramsey 2000). The contention is that a molecule has any number of appearances depending on the time scale of measurement. The shape of the molecule changes, for instance, as we zoom in using more refined instruments. As is described by Ramsey, while ammonia looks like a trigonal pyramid in IR spectroscopy, the molecule’s shape changes to two overlapping trigonal pyramids with one pyramid inverted with respect to the other if we use a more sensitive spectroscopic technique that distinguishes the rotation-vibration levels. And in the limits of perfect spectral resolution, the shape becomes spherical. Thus, whether the molecule has a particular shape is contingent on the way it is detected.[4] In other words a particular shape does not belong essentially to matter as a basic physical feature. Shape is not a primary quality, and cannot serve as the basis for justifying the classical model in stereochemistry. Admittedly, in cases where the time scale of physical measurement is of the same order as that of chemical reactions, the classical model of the molecule explains why molecules have the physical shape as they do, but not vice versa. Physical measurements of molecules (or the physical shape, which is the product of the measuring) do not give the molecular structure, nor justify it. This is in the first place owing to the fact that the concept of molecular structure is the product of the chemical investigation, which is distinct in purpose as well as in viewpoint from the physical investigation.[5] The concept of structure is based on empirical knowledge of chemical transformation. In the second place it may be ascribed to the fact that many of the physical analyses rest on the concept of molecular structure in analyzing data as is shown in the following. An unsymmetrical relationship between the structure and the shape of molecules is typically shown in the determination of the absolute configuration of chiral substances by using X-ray analysis.[6] The absolute configuration is determined by using so called anomalous X-ray scattering: when X-rays of a wavelength near the absorption edge of one of the atoms are irradiated, pairs of spots (Bijvoet pairs) that are related by the center of symmetry appear in unequal intensity. Then, provided that the molecular structure is known except for the absolute configuration, one can calculate the relative intensities of Bijvoet pairs for R and S isomers by comparison with an experiment performed with an authentic sample of known absolute configuration (Eliel et al. 1994, p. 113). The information about the structure is a prerequisite for the determination of the absolute configuration. In fact many of the physical techniques used for structural analysis are too naïve to tackle the many-body system in dynamic motion. It is here that the classical model of the molecule should be appreciated. The modal structure of the model has been known to provide a mostly adequate explanation of chemical phenomena so far examined though it cannot be proved true by physical measurements, for the reason described above. 5. Analysis of the classical model by the concept of affordanceAny effort to vindicate the classical model of the molecule by physical measurements is destined to fail because the molecular structure and the physical shape are models derived from different activities. Such an effort is likely to commit a kind of logical fallacy. Instead, it is the concept of the affordance that consolidates the ground on which the classical model of the molecule is supported. In this section I first introduce three concepts, that is, the concept of the affordance, the mereological fallacy, and the hinge, which are put together to constitute a tool kit for the philosophy of chemistry (Harré 2014). Then, I will apply these concepts to the problem raised and left unsolved in the previous section so as to make a claim that the classical model of the molecule is empirically adequate. The affordance is what is afforded by a hybrid being consisting of an experimenter, an apparatus, and the world to be investigated. For instance, chemical facts are taken as affordances of a hybrid specific to chemistry (i.e., a hybrid consisting of a chemist, a chemical laboratory, and substances). The point to be noted is that the affordance is ascribed to the whole of a hybrid, but not to its parts. It is a human being, but not the cerebral cortex, that thinks. It is called a mereological fallacy to ascribe an attribute of a whole to any of its parts. The three components are indispensable as a set for affording something meaningful to us. According to Harré, an apparatus is not a transparent window on the world. Nor are chemists’ practices the same as physicists’. Thus, the hybrid of world-apparatus-experimenter is at the core of the meaning of the vocabularies we use for describing chemical phenomena. It is much preferable from the empirical point of view that scientific arguments take into account not only products but also operations responsible for them.[7] A problem to be investigated by means of the concept of affordance is in what respects the classical model of the molecule is tenable. In other words, the question to be answered is why it is invalid to infer molecular structure from the data obtained by physical measurements. In contrast, it is permissible to infer molecular structure from observations of chemical reactions. We have to look for sound criteria according to which inferences are justified. The affordances specific to chemical practice are, for instance, products of chemical transformations (including the information about them), models of molecular structure, and so on. The models, which are taken as the constituents of what is to be analyzed, or substitutes for them, are inferred from chemical facts (that is, the products referred to above). We regard these inferences as legitimate if the products and the constituents share some basis for identity and individuation.[8] It is natural to require philosophical compatibility between these entities because models explain why chemical practices have the affordances they do. An inference about the reaction mechanism from the ratio of produced stereoisomers, as is often witnessed in chemical literatures, is legitimate in this respect. An inference of the shape of molecules from the products of physical measurements is likewise legitimate. On the other hand an inference about the structure from physical measurements is not justified because the criterion is not shared between them. The shape and the structure are attributes of different hybrid entities. According to Harré the evidence that an inference is legitimate comes from affordances, which are disciplined with respect to realists’ or heuristic interpretations by attention to hinge-practice and hinge-proposition pairs which incorporate the working metaphysics of an era.[9] Thus, if inferences (and the resultant models) are shown to be coherent with the dominant hinge via successful practices and unified propositional descriptions of a hypothetical mechanism, then the theory is regarded to be plausible. Based on these arguments it seems fair to claim that the classical model of the molecule is empirically adequate in that it is an essential part of stereochemistry, on which the causal nexus of chemical things and events have been established. However, it may be only from the chemical point of view that we can claim the adequacy of the classical model of the molecule. The reason why the affordance gives us confidence in what we are doing and believing is that it has established itself on an empirical basis and has entrenched itself in relation to hinge-practices and hinge-propositions. While we appreciate this on the one hand, we have to be careful with the risks it involves on the other hand. For instance, affordances specific to chemistry are likely to plunge into micro-reductionism (i.e., the view that chemical properties are wholly explicable in terms of intrinsic microphysical properties). Taking this fact into account, it is desirable to test models not only in the chemical context, but in much wider contexts. Enantiomorphism is a candidate with which to test the adequacy of the classical model of the molecule, because it may depend on the nature of space for its appearance. 6. Enantiomorphism and its philosophical implicationsStereoisomers are compounds that have the same sequence of covalent bonds and differ in the relative position of their atoms in space (Steitwieser and Heathcock 1976, p. 105). For instance, there are two stereoisomeric 2-iodobutanes that are non-superimposable mirror images. The relationship between the two isomers is the same as the relationship between right and left hands. The general property of handedness is called chirality. Thus, these two isomeric 2-iodobutanes are chiral molecules. Two compounds that differ in this way are called enantiomers. We say two isomeric 2-iodobutanes have an enantiomeric relationship to each other. The origin of the chirality of 2-iodobutanes is owing to the second carbon atom, to which iodine atom is attached. The second carbon of 2-iodobutane has four different groups. Such an atom is called an asymmetric carbon atom. When a molecule has one asymmetric carbon atom, it is always chiral. So much for the definitions of chirality and enantiomers. As was described in Section 4, the shape of molecules is a kind of dispositional property in that it changes its appearance according to the conditions of measurement. Therefore, the above definitions obtain only for molecules in a state expressible with the classical model of the molecule. It must correspond to the time scale in which usual chemical reactions in liquid phase take place because the classical model has been established based on empirical knowledge about chemical reactions. The question to be addressed in this section is whether enantiomorphy is intrinsic or extrinsic to the molecule. That is concerned with the question whether enantiomorphy depends on the nature of space. Confining the study to molecules under the usual conditions of chemical reactions, it will not be irrational to discuss the shape of molecules with respect to enantiomorphism. There is no way to tell whether enantiomorphy is intrinsic or extrinsic before examining the problem in detail. Le Poidevin claims that enantiomorphy is an extrinsic property of the molecule, and that it depends on the nature of space (Le Poidevin 2000). This claim is to be examined in detail below. ‘An intrinsic property of the molecule’ means that the property (enantiomorphy of the molecule in this case) does not logically depend on the properties, existence, or nonexistence of any object other than the molecule in question. On the other hand, ‘an extrinsic property of the molecule’ means that it logically depends on the properties, existence, or nonexistence of some object other than the molecule in question (for instance, the space in which enantiomers are embedded). The following thought experiment put forward by Le Poidevin will be helpful to imagine the relationship between shape and space, and the difference between the intrinsic and the extrinsic, as well. Think of a flat object in the shape of R, and a second object that is the mirror image of the first, both of which are embedded in a two-dimensional space. They are enantiomers to each other in this hypothetical space. They cannot be made to coincide by any rigid motions as long as they are confined to this space. However, if the space is a Möbius strip, an appropriate sequence of rigid motions will allow them to coincide. Alternatively, if they are permitted to move in a third dimension of space, that is, one of them is lifted off the flat space and turned over before replacing it again, the two objects coincide. This thought experiment suggests that, as a possible interpretation, enantiomorphy depends on the geometry of the space in which it is embedded, and is thus not an intrinsic, but an extrinsic property of the molecule. By the word ‘extrinsic’ Le Poidevin seems to imply from the beginning of his arguments that enantiomorphy is something indefinable within a chemical system, but something dependent on the nature of space. I do not see, however, a logical necessity to short-circuit to space before examining it in a wider context. The contention is that there are two types of extrinsic property; i.e., the first is an extrinsic property that owes to other objects, and the second is something dependent on the nature of space. The first type is common and ubiquitous in chemistry, and will thus be examined first. For instance, the ease with which a substance participates in chemical reactions depends on the properties of the other reactants, the presence or absence of catalysts, the properties of solvents, the temperature, and so on as well as on its own chemical properties. Oxidation-reduction reactions offer a typical example. The susceptibility of a substance to oxidation depends on the difference of oxidation potentials between the substances to be oxidized and reduced. Thus, it is extrinsic by definition. In fact, such is the case with chemical reactivity in general, because it can be defined not for a single reactant, but between reactants participating in a specific reaction. Likewise, enantiomorphy is a property to be defined not for a single isomer, but between a pair of isomers, suggesting that enantiomorphy is an extrinsic property in this sense. In contrast there is no evidence that suggests enantiomorphy being an extrinsic property in the other sense, that is to say, a property due to the nature of space. Actually, it may be too early to exclude this possibility at the present state of the argument. The possibility that it is the second type of extrinsic property is examined next. Enantiomers are distinct in absolute configuration, and hence incongruent with each other, whereas they show identical chemical reactivity toward achiral reagents.[10] For the sake of clarity, we discuss the problem in two-dimensional rather than three-dimensional space in the following. "Our orientation in a higher dimensional space toward some side of the manifold in which a chiral object is embedded prompts our inclination to call it left or right" (Nerlich 1994, p. 52). The following illustration put forward by Nerlich helps to understand his contention (Fig. 1). There is a thin vertical glass sheet to which the knees are confined and move rigidly around. They are in the sheet, but not on it. They are, as it were, the enantiomers in this two-dimensional space. Seen from one side of the sheet, a knee will be, say, a left knee. But move to the other side of the sheet and it will be a right knee. Thus, to call it right or left is an entirely fortuitous piece of naming. This story suggests that enantiomorphy is inseparably related to the geometry of the space. For instance, enantiomers incongruent in Euclidean space may be congruent in a non-Euclidean space. On a Möbius strip one enantiomer can be mapped onto its mirror image by a rigid motion around the circuit of the strip, and it is never enantiomorphic.
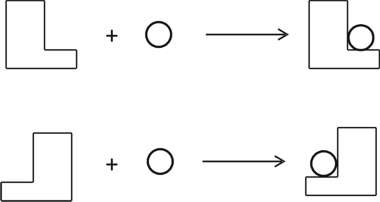
Figure 1. Two enaniomer knees. Enantiomers R (say, a knee on the right side, Fig. 1) and S (a knee on the left side) in two-dimensional flat space are incongruent with each other. The coupling or addition reactions of such R or S with achiral symmetric molecules (shown by a small circle in Fig. 2-a) provide compounds which are also incongruent on this flat space. But they will coincide if the space is non-orientable as a Möbius strip. On a non-orientable space the shape of the two objects will turn out to be the same. Hence, they are identical in chemical reactivity, and chemically indistinguishable. In contrast, the coupling reactions between R and R (or S and S), and R and S, provide diastereomers which are different not only in configuration but also in shape, and hence incongruent in any space as is shown in Fig. 2-b. This is the reason why diastereomers are chemically as well as physically distinguishable. This seems to suggest that enantiomorphism is an extrinsic property of the second type, but it is not necessarily so; a consistent argument can be made without recourse to the nature of space as shown below.
Figure 2. The reactions of enantiomers. Chemical reactivity is the tendency for a substance to participate in chemical reactions. For instance, whether or not a substance reacts, and how fast it reacts are both taken as questions concerned with chemical reactivity, which is related to the thermodynamic as well as the kinetic aspects of chemical reactions. Factors such as chemical composition, atomic linkage, the spatial arrangement of atoms, or combinations of these are responsible for the chemical reactivity of the molecule. These are constituents of the classical concept of the molecule, which assumes the influence of structure and the nature of each element on the chemical reactivity of the molecule (Ochiai 2013). We also know that the force of attraction (or repulsion) between reactants is inversely related to the square of the distance between them. Therefore, the difference in chemical reactivity between stereoisomers must be reduced to the difference in inter-atomic distances and bond angles unique to the local, intramolecular environment of each isomer. Enantiomers are identical in these respects, and hence they have to be chemically indistinguishable. The coupling or addition reactions of enantiomers with achiral substances provide a pair of products which are chemically indistinguishable because they are identical in these respects. Reactions between chiral substances, say, one with R configuration, and another with S configuration, provide compounds with a configuration representable as R-S. Its diastereomer with R-R configuration is different from the R-S in both inter-atomic distances and bond angles, and hence they are different in chemical as well as physical characteristics. These observations show that what makes a difference in chemical reactivity between enantiomers is the local environment of the kind described above.[11] So far we have examined enantiomorphism with and without reference to the nature of space. The intrinsic/extrinsic problem is, however, still open to further examination. We cannot say with certainty whether Le Poidevin’s claim is tenable or not. All we can say about enantiomorphism is that it is explicable by means of the classical model of the molecule as well. We do not see a necessity to invoke the special nature of space to understand enantiomorphism. One important implication revealed through these arguments is that the affordance specific to each science is not closed to possible tests from the viewpoints of other hybrids of world-apparatus-experimenter. This is further evidence to support the adequacy of the classical model of the molecule. While the real processes of chemical reactions may be not as clear-cut as is described in textbooks, the affordances specific to chemistry convince us that chemical reactions proceed basically as assumed. Otherwise it becomes far more difficult to give rational explanations for what we observe. But why do we find difficulties in explaining otherwise? Because it is the affordance that creates a view of the world in such a way as is described in the previous section, and one not inconsistent with the purposes of that science. Scientific facts are, therefore, nothing else but affordances, and hence distinct from the theory-free, brute facts. While ‘shape’ and ‘structure’, for instance, seem something physical, what are actually referred to by these words in chemical literatures, are concepts specific to chemistry. It is true that issues of stereochemistry lie on the boundary between chemistry and physics, but they should be so formulated and investigated as to meet chemical needs and from a chemical point of view. It is essential for the issues to be meaningful to us. Issues unable to be examined by chemical methods should not be regarded as chemical issues. Such might be the case with problems put forward by Le Poidevin concerning the geometry of space. Put it the other way around, it seems unlikely that the investigation of the chemical behavior of enantiomers will reveal the nature of space. Chemistry concerns transformations of matter, as is typically shown by the designing and synthesis of molecules. 7. ConclusionChemistry is governed by an action-related conception of knowledge, as described by van Brakel citing from Schummer (van Brakel 2000, p. 72). This point has been explicated recently by Laszlo who claims that the philosophy of chemistry needs to be primarily a philosophy of action (Laszlo 2014). Such a science of chemistry has long been subject to the criticism, however, that it is a complex system of miscellaneous know-how and empirical knowledge, but not an exact or proper science. Accordingly, Dirac declared that the essential part of chemistry can be reduced to mathematics (van Brakel calls this "the twentieth century culmination of Kant’s view"). Kant claimed that chemistry does not count as a proper science because it uses no mathematics. Chemistry that draws on laws of experience, i.e., mere regularities subject to Hume’s skepticism, is a "systematic art or experimental doctrine" at most (van Brakel 2006). However, Kant changed his view in his later life to acknowledge that much would be missed if one is stuck with only physics. What is unbeknownst to mathematical physics is to give an account of the variety of substances, a problem which chemistry has long addressed. Actually, in contrast to closure-seeking physics, chemistry is ever-expanding, or as is often stated, ‘chemistry creates its object’, a motto ascribed to Berthelot (Rocke 2001, p. 254). As to the last point, it is worth noting that such a state as an ever-expanding or metaphorically expressed as an ever-receding horizon could not be realized without the unique culture of chemistry, i.e., thinking with one’s hands. It is fair to say that chemistry is what chemists do. In conclusion we claim that stereochemistry, the science of molecular structure, holds good as far as it is concerned with chemical phenomena observed under usual conditions of chemical reactions. Notes[1] Weininger ascribes the relative neglect of dynamic aspects of chemical processes to the following: The theory of structure, which can be characterized as chemical architecture, contributed much to clear up the chaotic state of organic chemistry in the earlier decades of the nineteenth century, which is sometimes described as a ‘dark forest’ or ‘labyrinth’. Most evaluated in this context was van ’t Hoff’s hypothesis, which does not take into account the dynamic character of molecules, and enjoyed great success in solving the problem of stereoisomerism. In contrast, chemists in the nineteenth century were "not able to derive practical value from treating molecules as dynamic objects". Instead, they resorted to thermodynamics to attack chemical problems (Weininger 2000). [2] Hacking cites the episode that when his friend told him spraying super-cooled niobium balls with electrons decreases the electric charge of the niobium balls, he was convinced that electrons really exist, and this story made him a genuine realist (Hacking 2008, pp. 208-209). [3] R and S are symbols used for denoting the difference in absolute configuration of the two enantiomers. As to the absolute configuration, see note 6. [4] Considered from the viewpoint of quantum theory, ‘molecular structure’ is a consequence of environmental perturbations as is described in Section 2. And hence, on a time-scale in which the energy exchange between a molecule and its environment does not take place, or in the limit that a molecule is taken to be in a stationary state, a molecule cannot exhibit any structural property including optical activity (Woolley 1978). The spectroscopic observation seems to support this consideration. [5] Chemical practices require the classical concept of the molecule which is taken as a map to show possible sites and possible types of transformation for the designing of molecules. Information on the spatial arrangement of atoms (or nuclei) without chemical bonds is useless for this purpose (Ochiai 2013). [6] The configuration of an asymmetric carbon atom is the specification of the relative spatial placement of the four groups attached to that carbon. The absolute configuration specifies their order in such a way as to distinguish the two enantiomers. The system for specification of the absolute configuration is defined as follows; the four atoms attached to the asymmetric carbon atom are arranged in a sequence of decreasing priority (Order of decreasing priority is defined precisely). A three-dimensional model of the isomer to be named is viewed from the side opposite the group of lowest priority, and the sequence of the other groups is noted as clockwise or counterclockwise. When the sequence is clockwise, the symbol R is used to denote the configuration. When it is counterclockwise, the symbol S is used (Hendrickson, Cram & Hammond 1970, pp. 203-206). [7] The analytical power of the concept of the affordance is shown in the story that the particle-wave duality can be resolved by treating the seemingly contradictory attributes of basic material beings as paired affordances linked to distinct world-apparatus- experimenter set-ups. [8] Harré explains that atoms and molecules, of which they are presumed to be constituents, share a spatio-temporal basis for individuation and identity. This criterion pattern is not shared with electrons, particularly spatio-temporal continuity in relation to identity. [9] A hinge is something of which we are certain like one’s own gender. In other words, it is an a priori foundation for conceptual and material practices such as causes precede their effects. Hinge practices are the core activities of forms of life, and sometimes expressed in hinge propositions. When one tries to formulate a hinge proposition, one finds that it is a putative empirical fact which no one has ever doubted or brought into question. [10] Provided that there is no silent or causally impotent property intrinsic to the molecule, this suggests that enantiomorphy is extrinsic to the molecule. [11] The difference between enantiomers discussed here is distinct from the one referred previously as an extrinsic property of the first type. While in the latter the focus is on the relative reactivity, the present discussion is concerned with the origin of the difference in reactivity. ReferencesCartwright, N.: 1983, How the Laws of Physics Lie, Oxford: Clarendon Press. Chakravartty, A.: 2007, A Metaphysics for Scientific Realism, Cambridge: Cambridge University Press. Eliel, E.L.; Wielen, S.H. & Mander, L.N.: 1994, Stereochemistry of Organic Compounds, New York: Wiley. Giere, R.N.: 1988, Explaining Science; A Cognitive Approach, Chicago: University of Chicago Press. Hacking, I.: 2008, Representing and Intervening (22nd ed.), Cambridge: Cambridge University Press. Harré, R.: 2014, ‘New Tools for Philosophy of Chemistry’, Hyle: International Journal of Philosophy of Chemistry, 20(1), 77-91. Hendrickson, J.B.; Cram, D.J. & Hammond, G.S.: 1970, Organic Chemistry, International Edition, Tokyo: McGrow-Hill Kogakusha. Laszlo, P.: 2014, ‘Chemistry, Knowledge Through Actions?’, Hyle: International Journal of Philosophy of Chemistry, 20(1), 93-116. Le Poidevin, R.: 2000, ‘Space and the Chiral Molecule’, in: N. Bhushan & S. Rosenfeld (eds.), Of Minds and Molecules; New Philosophical Perspectives on Chemistry, Oxford: Oxford University Press, pp. 129-142. Nerlich, G: 1994, The Shape of Space (2nd ed.), Cambridge: Cambridge University Press. Ochiai, H.: 2013, ‘The Logical Structure of Organic Chemistry and the Empirical Adequacy of the Classical Concept of the Molecule’, Hyle: International Journal of Philosophy of Chemistry, 13(2), 139-160. Ramberg, P.J.: 2003, Chemical Structure, Spatial Arrangement, Hampshire-Burlington: Ashgate. Ramsey, J.L.: 2000, ‘Realism, Essentialism, and Intrinsic Properties’, in: N. Bhushan & S. Rosenfeld (eds.), Of Minds and Molecules; New Philosophical Perspectives on Chemistry, Oxford: Oxford University Press, pp. 117-128. Rocke, A.J.: 2001, Nationalizing Science; Adolphe Wurz and the Battle for French Chemistry, Cambridge/MA: MIT Press. Streitwieser, J.A. & Heathcock, C.H.: 1976, Introduction to Organic Chemistry, New York: Macmillan. van Brakel, J.: 2000, Philosophy of Chemistry, Leuven: Leuven University Press. van Brakel, J.: 2006, ‘Kant’s Legacy for the Philosophy of Chemistry’, in: D. Baird, E. Scerri & L. McIntyre (eds.), Philosophy of Chemistry; Synthesis of a New Discipline, Dordrecht: Springer. Weininger, S.J: 2000, ‘Butlerov’s Vision: The Timeless, the Transient, and the Representation of Chemical Structure’, in: N. Bhushan & S. Rosenfeld (eds.), Of Minds and Molecules; New Philosophical Perspectives on Chemistry, Oxford: Oxford University Press, pp. 143-161. Woolley, R.G.: 1978, ‘Must a Molecule Have a Shape?’, Journal of the American Chemical Society, 100, 1073-1078. Ochiai Hirofumi: |
 (a)
(a)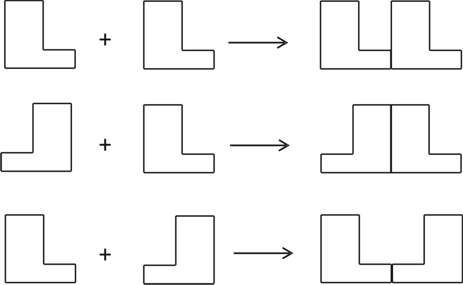 (b)
(b)