http://www.hyle.org
Copyright © 2014 by HYLE and Lukasz Lamza
Six Phases of Cosmic ChemistryLukasz Lamza*
1. IntroductionThe steady development of astrophysical and cosmological sciences has led to a growing appreciation of the continuity of cosmic history throughout which all known phenomena come to being. This also includes chemical phenomena and there are numerous theoretical attempts to rewrite chemistry as a ‘historical’ science (Haken 1978, Earley 2004). It seems therefore vital to organize the immense volume of chemical data – from astrophysical nuclear chemistry to biochemistry of living cells – in a consistent and quantitative fashion, one that would help to appreciate the unfolding of chemical phenomena throughout cosmic time. Although numerous specialist reviews exist (e.g. Shaw 2006, Herbst 2001, Hazen et al. 2008) that illustrate the growing appreciation for cosmic chemical history, several issues still need to be solved. First of all, such works discuss only a given subset of cosmic chemistry (astrochemistry, chemistry of life etc.) using the usual tools and languages of these particular disciplines which does not facilitate drawing large-scale conclusions. Second, they discuss the history of chemical structures and not chemical processes – which implicitly leaves out half of the totality of chemical phenomena as non-historical. While it may now seem obvious that certain chemical structures such as aromatic hydrocarbons or pyrazines have a certain cosmic ‘history’, it might cause more controversy to argue that chemical processes such as catalysis or polymerization also have their ‘histories’. The purpose of this article is to:
The problems standing before such a venture are obvious and numerous. First of all, there are still considerable gaps in our understanding of cosmochemical history, the most dramatic of which is probably the transition from non-living to living systems, abiogenesis. However, the general ‘story of the universe’ seems to be secured and there is ample observational data to support it. This concordant cosmic history, backed up by preliminary quantitative estimates, forms the background for the analysis presented below and is introduced in Section 3. Second, there is no generally agreed-on language that would serve to express the growth of chemical complexity through time. In other words, if one were to present the vast universe of chemical phenomena in an evolutionary fashion, the assembly of a list of such phenomena would be the first obstacle. In order to actually produce a systematic review of cosmic chemical evolution, we may not limit ourselves to mentioning whatever comes to our mind. Here, I wish to present a solution, admittedly tentative, to this second problem, by borrowing a list of chemical phenomena from the Universal Decimal Classification (UDC 2005): a library cataloguing system. UDC is a detailed hierarchical system for classifying library resources and contains a list of ca. 2700 topics in chemistry which can be read at any level of detail. Although it may seem an odd and risky choice to employ library catalogues for the purposes of quantifying natural phenomena, I believe that this is in fact a rational decision and works exceedingly well as a first approximation. The UDC system, its basic properties, and the rationale for its use are discussed below in Section 2. 2. Why use library classification systems?In order to appreciate the growth of natural complexity throughout the cosmic evolution, it does not simply suffice to tell the cosmic story. A quantitative element, if only tentative or even semi-quantitative, seems necessary. A good illustration of that principle is paleontology, where evolution of life is often quantified by the number of extant taxa of given rank (genera, families, orders, classes, phyla) throughout geological time (see e.g. Valentine 2004, Rohde & Muller 2005). At first sight, the method may seem fully quantitative, yet it is not, because it is not based on a robust method of attributing ranks to taxa. For instance, there are no good reasons why there are, say, 3 orders of leeches (class Hirudinea), 13 orders of monocot plants (class Liliopsida) and 45 orders of bony fish (class Osteichthyes) and, in fact, why these three groups have been assigned the rank of class. The number of orders (or that of any other high rank) does not easily correlate with species richness or any other obvious measure, and is rather a function of tradition, convenience, and, increasingly, phylogenetics – which leads, in the opinion of at least some biologists, to even less balanced classification systems (Cavalier-Smith 1998). Yet, any discussion of the history of biological complexity based on order counts would attribute to the emergence of bony fish exactly 15 times more ‘power’ than to the emergence of leeches. Have bony fish as a group really been measured to be 15 times more complex/important than leeches? This example is intended to demonstrate two things. First, there is no real hope of developing a ‘natural’ or, even more so, ‘objective’ list of phenomena of given type at a defined level of detail that would enable us to ‘measure’ the growth of complexity of different groups of natural objects. Second, even an intuitive measure that is created simply for convenience may lead to valuable insights, as demonstrated by the ubiquitous and creative use of the above-cited biological diversity measure in paleontology (for a broad sample of such diagrams, see Valentine 2004). The ‘list of chemical phenomena’ used throughout this article is very much similar to the ‘list of living organisms’ that one would get by choosing a particular taxonomical rank (e.g. order) and listing all currently recognized biological groups of that rank. Here, out of numerous other possibilities, UDC’s class names are used. Universal Decimal Classification was first published in 1905 and has been steadily modified and extended since then to now include ca. 69000 hierarchically ordered classes. To get a feel of the system and its structure, let us note the class names of successive levels of hierarchy leading to the most detailed class that would be used to tag library resources concerning anthracene:
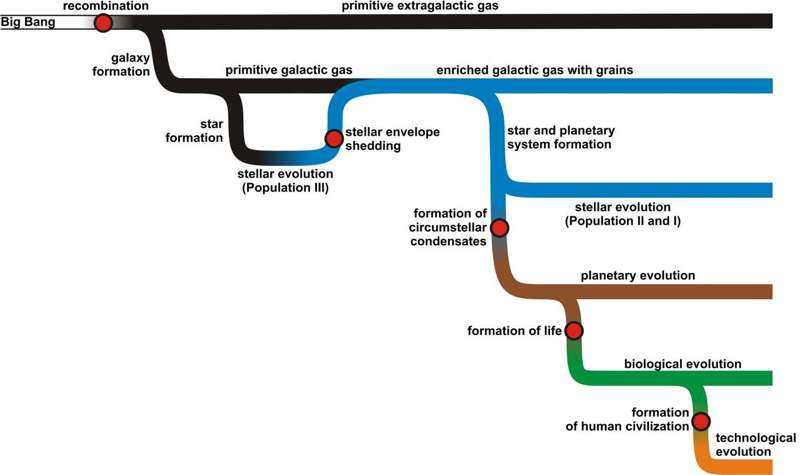
Here and below, the name ‘level-n class’ will denote the class whose class number contains n digits. The similarity to the biological classification system is striking and, in fact, a taxonomical treatment of living beings forms a subset of UDC. Also, one encounters here the seemingly insoluble problem of rank assignment and handling the complexity across disciplines. Above, we have shown how three distantly related groups of organisms were assigned the rank of classes and subdivided into a seemingly arbitrary number of lower rank subgroups (orders). In UDC one witnesses the exact same issue. The level-V class ‘552.55 Siliceous rocks’ contains 3 level-VI subclasses, the class ‘547.67 Trinuclear systems’ contains 6 such subclasses and there are 9 subdivisions of the class ‘591.43 Organs of animal nutrition’. Is there even a slightest hope of determining the appropriate ranking system? I leave this issue open as an interesting question on its own. Although I realize that this short exposition may not suffice to convincingly present the case for using the number of library catalogue entries as a proxy for natural complexity, I hope that the example of paleontology shows at least that analogous procedures have been successfully used in other disciplines. Also, the (intended) exhaustive nature of the system should be noted – just as any living creature should in theory have a place in the taxonomical system of biology, any book or article concerning any aspect of chemistry should have its place somewhere within the UDC system. Even if this noble vision is forever out of reach, the use of a library catalogue is intended as a simple measure against the possibility of writing a natural history of chemical evolution based simply on those chemical phenomena that a particular author had in mind at a particular time. To use the biological metaphor once more: even if the list of all currently recognized orders of living organisms does not represent all of nature’s biological richness, it is certainly a convenient approximation to the current state of knowledge. A broader critical perspective on the method used is found in Section 7. In the following, all 176 level-V subclasses of four level-III classes relevant for the study of the growth of natural chemistry (‘544 Physical chemistry’, ‘546 Inorganic chemistry’, ‘547 Organic chemistry’ and ‘577 Material basis for life. Biochemistry. Molecular biology. Biophysics’) are used. Two level-III classes (‘542 Practical laboratory chemistry’ and ‘543 Analytical chemistry’) and 2 level-V classes (‘544.18 Quantum chemistry’ and ‘544.42 Formal kinetics’) of chemical nature were excluded, because they specifically describe human activities related to chemistry, and not natural chemical phenomena. Also, for this group the translation of class names to names of chemical phenomena and a physico-chemical interpretation of their novelty are highly non-trivial – which will be commented further in Section 4.6. Some of the resulting classes (given, for clarity, without class numbers and in plain text, from now on being treated as names for chemical phenomena, not names for subfields of science[1]) are: atomic and molecular spectra; combustion and explosion; plasma chemistry; colloid chemistry; phosphorus and its inorganic compounds; rare earths; unsaturated hydrocarbons; phenols and aromatic alcohols in general; trinuclear systems. The purpose of this study is to track the appearance of entities and processes denoted by these class names throughout the cosmic history with a proper appreciation of the relevant cosmological, astrophysical, and geological context. 3. The big picture of cosmic evolutionThe presentation of the development of chemical phenomena throughout cosmic time will follow in Section 4, but first some preliminary explanations are in place. The big picture of cosmic evolution that serves as a background for chemical evolution is presented in Figure 1. All elements of the scenario described below are now generally agreed on and well corroborated by observations. The interested reader may find more details on the general history of the cosmos in numerous sources (e.g. Liddle 2003, Pagel 1997, Tolstikhin & Kramers 2008). The terms in boldface refer to the terms used in Figure 1. The universe started out in a state precluding any form of chemistry, with temperatures not only exceeding ionization thresholds, but also levels enabling the existence of atomic nuclei in the first place. The gradual cooling of that prestellar gas led to the emergence of rudimentary chemistry (based on H, He, and Li only) and the collapse of matter into overdensities which eventually led to galaxy and star formation. The gas that did not participate in galaxy and star formation (primitive extragalactic gas) is a largely unstudied reservoir, although it slowly becomes observable (Simcoe et al. 2012). The stellar evolution of first stars (termed, slightly confusingly, Population III) led to the nucleosynthesis of all heavy elements and culminated in the ejection of enriched gas (stellar envelope shedding) that condensed into a small but solid population of chemical and mineralogical species. From this moment on, the galactic gas is being steadily cycled through the nuclear machinery of stars, leaving behind an increasingly more enriched interstellar gas with grains and a growing population of stellar remnants (not pictured). Our Sun is one of those stars that formed from a gaseous reservoir containing not only the dominating H+He component, but also a small admixture of heavier elements which initiated planetary system formation. Around the young star condensation of matter occurs, forming a reservoir that might be termed circumstellar condensates. This includes comets, asteroids, planetoids, planets and dwarf planets, their satellites, interstellar dust particles, and other objects orbiting the Sun, primarily composed of condensed matter and a primitive stellar-like gas. Planetary evolution ensues. On at least one planet chemical systems turn into living systems, initiating the phase of biological evolution. Human civilization is but one of the numerous ‘special cases’ of biological evolution; from a chemical perspective a rather spectacular one, too – but that will be discussed in further detail in Section 4.5.
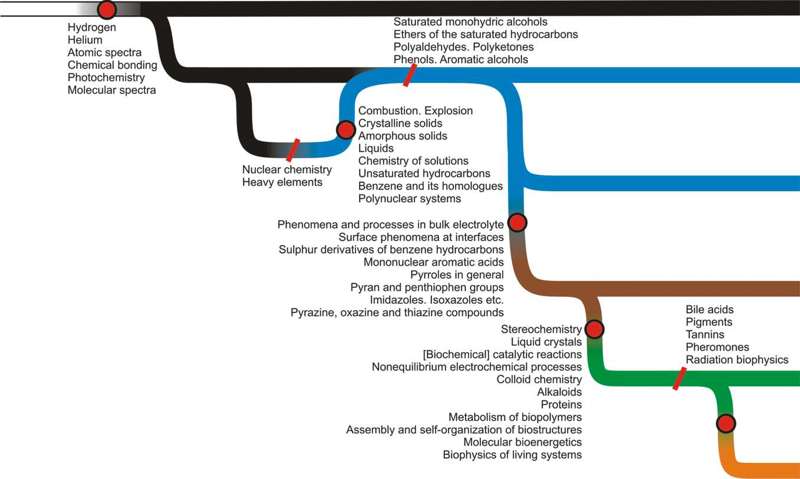
Figure 1: The big picture of cosmic evolution, time runs from left to right. Six phases of cosmic chemical evolution described in the article are symbolized by six colors and red spots indicate areas and processes in the Universe that lead to the emergence of new such phases. More information in text. This complex history may be schematically divided into six segments (‘phases’), sequentially discussed in Section 4. Each phase is initiated by a dramatic, intensive, and usually rapid pulse of chemical creativity and each leads to a closed and mostly static repertoire of chemical phenomena. What is especially important, and graphically illustrated in Figure 1, is that the new chemical revolutions always concern only a small portion of cosmic matter and that up to this day there are ‘primitive’ reservoirs in the Universe that are relatively simple and do not demonstrate chemical phenomena typical for more ‘advanced’ chemical phases. This is yet another similarity between chemical and biological evolution, where the evolution of new biological groups does not necessarily lead to the extinction of the ones from which they evolved. 4. The six phases of cosmic chemistryIn the following section, phrases in boldface refer to terms present in the UDC classes under consideration or in their subclasses. Some results discussed in this section are presented graphically in Figure 2.
Figure 2: The appearance of major chemical innovations throughout cosmic history. The ‘flow diagram’ used as background is described in Figure 1. All names come from the UDC. More information in text, including the discussion of possible controversies or uncertainties and the references to the relevant literature. 4.1. The Phase of No Chemistry: From the beginning to recombinationThe universe began with no chemistry whatsoever. The gradual cooling of matter led to a series of transformations, the most important of which, at least from the perspective of this article, is the phase of primordial nucleosynthesis which took place within the first three minutes of Big Bang. This Big Bang Nucleosynthesis is responsible for the well-known mixture of primordial gas with a mass proportion of hydrogen to helium (H:He) of approximately 0.75:0.25 and a tiny admixture of lithium and an even tinier of heavier elements. (All elements heavier than helium are in cosmology called metals, hence metallicity as the measure of the concentration of metals). Because of high temperatures, the matter was still fully ionized and no chemistry sensu stricto was present. 4.2. The Phase of Prestellar Chemistry: From the recombination to the first starsAbout 300,000 years after Big Bang, recombination took place and first neutral atoms appeared. This enabled collisional or radiative excitation, de-excitation and ionization with concurrent absorption or emission. This in turn brought atomic spectra (including the astronomically important hyperfine emission of H at 21 cm) to the radiation field. This rudimentary chemistry, in a rare and hot gas, with ambient temperatures around 2,000-3,000 K, persisted for only a couple million years. Around 2-5 million years after Big Bang, the first molecules started to form. Given the simple elemental composition,[2] this included H2 (and H2+, plus HD and HD+), LiH (and LiH+) and – which may be more surprising – a microscopic amount of HeH, whose relative abundance was estimated to be ca. 10-14 (Maoli et al. 1994). The first appearance of covalent chemical bonding also brought rotational and vibrational spectra. Note that the repertoire of vibrational spectra included only a tiny subset of known modes because the largest molecules at that time were two-atomic (no scissoring, wagging, twisting, etc.). This mostly neutral gas slowly began to collapse and about 30-50 million years after the Big Bang massive haloes (105-106 MΘ, where MΘ is the mass of the Sun) formed. These haloes, which can be thought of as the building blocks of protogalaxies, separated from the rare surrounding gas that would not participate in further chemical revolutions – which is presented graphically in Figure 1 as the first divergence between the primordial extragalactic gas and the primitive interstellar gas. Although this almost-zero-metallicity gas has not yet been unequivocally observed, promising signs of such detection do exist (Simcoe et al. 2012). Based on tentative cosmological calculations, up to 90% of all (baryonic) matter may be currently tied up in such an extragalactic gas of negligible metallicity (Fukugita et al. 1998, Fukugita & Peebles 2004) typical for the Second Phase – although such calculations should be treated with great care. The haloes soon fragmented into the first population of stars, termed for historical reasons Population III stars. 4.3. The Phase of Galactic Chemistry: From the first stars to planetary systemsThe following stages of cosmic evolution are all related to the star formation cycle, where galactic gas of suitable density and composition undergoes gravitational collapse, forms a star accompanied (in case of later populations of stars, symbolized in Figures 1 and 2 by the second blue loop) by a planetary system, and is finally either quietly extinguished (in case of very low mass stars) or more or less violently expelled (in case of heavier stars) leaving behind a compact remnant. These local events are in stark contrast to the global events described in the previous section – all the stages of stellar and planetary evolution, with the emergence of all related chemical phenomena, happen all the time in billions of slightly different variants throughout the universe. From the chemical point of view, the most important process in stellar evolution is nucleosynthesis – responsible for the proportions of elements produced – and the late stages responsible for the return of those elements to the interstellar medium with the concurrent creation of a considerable richness of chemical and mineralogical species. Because of the high temperatures of stars, even after heavy isotopes are synthetized the resulting gas is chemically ‘primitive’ until it cools down below the recombination threshold, analogous to the above-mentioned situation during the Big Bang. Stellar evolution is a vast and rich area of study, but for the purposes of this article two basic observations seem most important:
It is in stellar envelopes and in the interstellar medium, where a considerable proportion of known chemical processes and classes of compounds first appears. Out of 66 classes of organic molecules found in the UDC, 28 (42%) have been detected in the interstellar medium (for a recent review of astrochemistry, see e.g. Shaw 2006). All compounds mentioned in round brackets belong to this group. On the oxygen side, various oxides form, including compounds of N, S, and P, but also mineral-forming oxides, especially Al2O3 (corundum) and TiO2. These are later incorporated into minerals in a complex sequence of chemical and physicochemical processes, leading through rare mineral species such as gehlenite Ca2Al[AlSiO7] and akermanite Ca2Mg[Si2O7] to more well-known ones such as enstatite MgSiO3 or diopside CaMgSi2O6 (Davis & Richter 2003). Tiny grains of material incorporated into meteorites but formed before the Solar System – so-called presolar grains – also contain spinel MgAl2O4, olivine (Mg,Fe)2SiO4, and other major rock-forming minerals. Iron is partly incorporated into oxide minerals, but mostly forms a metallic alloy with nickel (known as kamacite or taenite, depending on proportions). Some numerical simulations (Lattimer et al. 1978) suggest that this sequence of mineralogical processes involves certain mineral phases for which the ambient temperature is above the liquidus – these should for a short period of time form tiny droplets: the first liquids in the Universe. On the carbon side, there are chain radicals and ions (C8H-, C5N-, and such) and saturated or unsaturated hydrocarbons (methane to hexane, acetylene, triacetylene) and later their simple derivatives, such as ethers (e.g. dimethyl ether), monohydric alcohols (methanol, ethanol), carboxylic acids (formic acid) and cyclic hydrocarbons, both aromatic (benzene) and alicyclic (cyclopentane), likely including their derivatives such as phenols. Further reactions between benzene and its derivatives lead first to the formation of binuclear and trinuclear aromatics, later polycyclic aromatic hydrocarbons (PAHs) and their weakly bound aggregates, then particles of soot and microscopic particles of graphene and graphite. Also, carbides such as SiC, TiC or MoC can be found in spectra of many giant stars and later become condensation nuclei and cores of aggregating interstellar grains. Naturally, grains of ‘pure’ carbon also form, consisting of graphite or diamond, depending on conditions. This growth of solids is accompanied by a fascinating transition from line to continuum spectra which can be thought of as an illustration of the transition from chemistry to solid state physics and is beautifully illustrated by the evolving spectra of PAHs of increasing size (Bakes & Tielens 1994). The whole process involves, for the first time, the (almost) full richness of reaction types (addition, elimination, substitution, rearrangement) and mechanisms (e.g. multistage reaction with highly reactive intermediates), plus related physicochemical processes (nucleation, formation of solutions). Metallic bonds (hinted in metallic clusters and fully developed in alloys) and weak bonds (e.g. in stacking interactions between PAH molecules) emerge for the first time which – accompanied by the above-mentioned enrichment in vibrational modes – leads to a more complex energy landscapes of molecules and the resulting growth in the complexity of spectra. In the high-energy environment typical for the late stages of stellar evolution, photochemical and radiochemical processes become important. Finally, the resulting mixture of gaseous and solid-state products cools down and is incorporated into the interstellar medium. There, on the scale of hundreds of millions of years, they attain equilibrium with the fluctuating radiation and cosmic ray field. The interstellar grains undergo slow evolution, reaching through collisions and aggregations an equilibrium size distribution with a maximum at about 100 nm and not exceeding 1-2 µm (Tielens 2010, p. 159). The surfaces of grains host solid surface chemistry, including first instances of rudimentary catalysis such as the very effective surface-catalyzed formation of H2 from H (Dyson & Williams 1997, p. 72). Because of the importance of gas cooling to astrophysical structure formation, this particular catalytic reaction is of prime importance to astronomy. Currently, about 23% of galactic mass is in the form of interstellar medium, 63% resides in active stars, and 14% in stellar remnants, with only a negligible amount (0.03%) in planetary systems (Fukugita et al. 1998). Because stars themselves host only a very limited repertoire of non-specific chemical phenomena (see e.g. Sinha 1991), to complete the ‘chemical picture’ of galaxies at the Third Phase, only stellar remnants are left. Their interiors are too dense and too hot to host any form of chemistry, and their surfaces and atmospheres host a poor-known repertoire of chemical phenomena, likely but not necessarily similar to ones found in normal stars (Gaur et al. 1988). However, the effect that stars and stellar remnants have on their environment is not to be underestimated from a chemist’s point of view. Just recently, an entirely new class of chemical bonding was implicated in ultra-strong magnetic fields such as those around white dwarfs (Lange et al. 2012). Any census of chemical processes or entities relevant for this phase of cosmic evolution should leave some place for the likely unusual high-energy phenomena related to compact objects. Had for some reason stellar formation ceased after Population III stars, the above-mentioned suite of chemical processes would be all there is in the universe. Luckily, further generations of stars are steadily created up to the present day. These, however, incorporate not only the bare primordial gas, but also the ‘metals’ that condense, aggregate, and ultimately form planetary systems. 4.4. The Phase of Planetary Chemistry: From planetary systems to the emergence of lifePlanetary systems are, in astrophysical scale, minor additions to stars. In the current mass budget of the Solar System, only 0.14% of mass belongs to the planetary system, the rest being locked up in the Sun (calculated from the data in Cole & Woolfson 2002). After accounting for the fact that most of the planets’ total mass rests in the gas giants’ gas – which has an essentially stellar composition – there remains only a tiny admixture of ‘heavy’ elements to form all the ices, rocks, and metals and the fantastic abundance of soft matter and chemical systems that we know from Earth. Let us quickly review what we know about the growth of chemical complexity that occurred with the formation of our planetary system. First, looking from the perspective of the (largely organic) ices formed during the Third Phase, through their irradiation, heating, and fractionation many large groups of organic compounds appeared for the first time, especially numerous heterocycles – pyrroles and pyrans (Cody et al. 2011), pyrazines, imidazoles, and carbazoles (Remusat et al. 2005), aromatic acids (Martins et al. 2006) and alicycles including cyclohexane (Pilling et al. 2012) and spiranes (Whittet 1997, p. 69). Life, from a chemical perspective, may basically seem like a very specialized and incredibly innovative segment of that chemistry – this will be discussed in the following Section. Second, looking from the perspective of the ‘rocks’, from the first moments the interstellar gas is heated by the young star, novel minerals and their associations form (Davis & Richter 2003). Thus, in short time, the initial repertoire of ca. 15 minerals found in interstellar grains grows to the estimated 250 present in primitive meteorites that did not go through planetary evolution (Hazen et al. 2008). From the earliest stages of planetary volcanism certain geologically interesting molecules are created, including the infamous chlorofluorocarbons (CFCs) responsible for ozone depletion in the 1970s and 1980s and other halogeno derivatives of hydrocarbons (Gribble 1994) which are present in volcanic gases. Later stages of geological evolution bring mostly mineralogical innovations which, because of their spectacular nature, deserve a short mention. It is estimated that from the 250 or so minerals present at the formation of Earth, the planet’s mineral repertoire grew to a stunning 4300 (Hazen et al. 2008). 4.5. The Phase of Biological Chemistry: From the emergence of life to humansOut of the 176 chemical phenomena distinguished for the purposes of this article, 43 are specific for the biological world. This may be little or a lot, depending on the perspective. Lacking a definite theory of abiogenesis, we may not safely discuss the amount and scope of physicochemical innovation necessary for the creation of life, but several general observations remain valid and several main biochemical novelties are well known. Let us list all the ‘usual suspects’ which can be found in any cell biology or biochemistry textbook. First of all, there are three main classes of complex biopolymers specific to biology: proteins, carbohydrates and nucleic acids. There are also other well-known large classes of biomolecules, either chemically well-defined, such as lipids and alkaloids, or ill-defined, such as pigments or hormones. Several smaller groups of compounds are found only in certain groups of living organisms: sterols are typical for eukaryotes and as such are sometimes used as biomarkers in paleontology;[3] lignins and tannins are plant-specific; chitin is present only in fungi and certain groups of animals and collagen in animals only. An amusing example of the evolution’s ability to penetrate unusual segments of chemical creativity is the emergence of the ability to produce ultrasounds in certain groups of marine mammals which induces a range of novel biomechanical and sonochemical effects (Krasovitski 2011). Second, the formation of life was related to a plethora of new chemical and physicochemical processes and properties, such as stereoselectivity[4] or the development of disperse phase and colloid chemistry. Lipid membranes are a rare example of natural liquid crystals. Biological systems exhibit high degree of control over polymerization and depolymerization under various chemical regimes. Cellular metabolism requires high-specificity catalysis including first true organic enzymes (unlike the generally low-specificity catalysis exhibited by mineral surfaces), exhibits instances of autocatalysis (e.g. in hammerhead ribozymes) and is based on a tightly controlled network of chemical cycles and pathways. Finally, the coordinated oxidation-reduction reactions in living cells and the very existence of ion pumps can be interpreted as electrochemistry. 4.6. The Phase of Human ChemistryThe purposeful activity of humans leads to the progressing penetration of all imaginable avenues of chemistry, from our first experiments with fire to modern analytical chemistry. On the structural side, a bewildering array of atomic, molecular, and supramolecular creations has been prepared in human laboratories whose appreciable stability or even the very existence seems very unlikely in nature. This is usually achieved by attaining right temperatures (especially ultralow temperatures), ultrapure substances, or protective non-reactive atmospheres, whereas in typical natural environments thermal fluctuations, defect-causing admixtures or a highly reactive environment would stop these phenomena from manifestation. Out of the plethora of possible examples, this is a small selection just from the previous year selected to represent different levels of chemical organization, from nuclear and atomic, through molecular and macromolecular to biophysical:
On the process side, all analytical techniques and tools of chemistry are obviously human inventions – from spectroscopy and mass spectrometry, to chromatography and gel electrophoresis. It is however disputable whether basic physico-chemical processes utilized in such methods are not at work throughout the universe. For instance, the differential action of magnetic fields on ions of different charge – the physical basis for mass spectrometry – is clearly a process expected to be active in many astrophysical settings. It would be probably much harder to find a natural equivalent of gel electrophoresis, where a heterogeneous population of particles is separated in a homogeneous medium through the action of an electric potential. The complicated nature of chemical technology, which involves the application of straightforward physico-chemical processes in complex and innovative procedures, would require a separate study on its own. 5. The similarity of the cosmic chemical transitions to phase changesAs already hinted in Section 3, the six phases described above are separated by short-lived episodes of intensive change which may be likened to phase changes. This should probably not be seen as surprising, as thermodynamics often permeates discussions of cosmic evolution (e.g. Prigogine & Stengers 1984, Kaufmann 1996, Schneider & Sagan 2006).
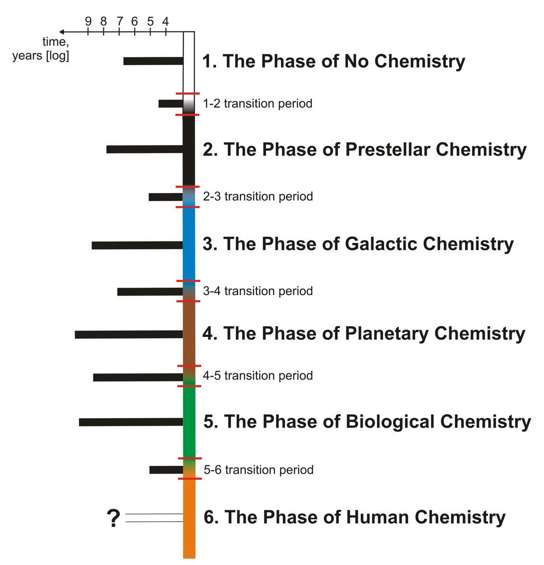
Figure 3: Summary of all major phases of cosmic chemical evolution with corresponding transition periods. The duration of these phases and ‘phase transitions’ is indicated by the length of the black bars. More information in text. Four properties of such transition periods are especially suggestive of a similarity to classic phase changes known from elementary thermodynamics:
Let us take a look at these properties in some detail. 5.1. The rapidity of transitionsThe major constituent of the 1-2 transition – the transition[6] from the H+ to the H-dominated Universe – took about 1.6·104 years (Stiavelli 2009, p. 12), while the preceding Phase of No Chemistry lasted for about 4·106 years and the following Phase of Prestellar Chemistry lasted for about 5·107 until the first stars collapsed (Stiavelli 2009, p. 24). Further phases, as it has already been indicated, do not simply occur successively at well-defined moments of cosmic history, but are being constantly initiated locally, so one cannot determine their duration unambiguously. One cannot say, for instance, how long the Phase of Planetary Chemistry was, because planets still evolve after the formation of life. However, all the processes involved happen on typical timescales and the transition periods have their typical durations. A natural timescale for the evolution of stars ranges from 107 to 1010 years. The 2-3 transition occurs during the late stages of stellar evolution and it takes about 105 years from the moment heavy elements are first ejected into cooling stellar envelopes to the time when they have mostly condensed and reacted with each other (Herwig 2005). The galactic chemistry evolves on a timescale similar to that of the stellar evolution: for instance, it takes Milky Way’s interstellar gas around 5·107 years to travel between two star-forming regions which marks episodes of increased chemical enrichment, compression, heating, and irradiation (Pagel 1997); interstellar grains have lifetimes on the order of 108 years (Jones 2004) and global enrichment of interstellar medium’s metallicity takes ca. 109 years (Lineweaver 2001). Thus, 108 years will be assumed here as a natural timescale for this phase. The interstellar medium undergoes the 3-4 transition during the formation of planetary systems and it takes about 107 years from the moment interstellar matter enters the protoplanetary disk to the time when most chemical and mineralogical processes mentioned in Section 4.3 have occurred and planets are essentially formed (Trieloff & Palme 2006). It is more difficult to put a time limit on the Phase of Planetary Chemistry and the Phase of Biological Chemistry. We do not know how long planets and life can evolve, but this seems largely dependent on the timescale of their central stars’ lives. Earth’s chemistry has evolved unimpeded for ca. 4.6·109 years and the biosphere only slightly shorter, so, for lack of more data, this lower limit may be used as a first approximation. We have no knowledge whatsoever of how long it took Nature to perform the 4-5 transition (abiogenesis). It is generally assumed that first signs of life appeared ca. 3.5-3.8·109 years ago (see e.g. Wacey et al. 2011), and only 4·109 years ago was our planet cool enough and safe from planetary-scale impacts to permit a stable hydrosphere (Williams 2007); thus, 5 108 years for the whole transition is a reasonable upper limit. If the rapid nature of large chemical transitions is in fact a regularity of Nature, it could be assumed that abiogenesis took considerably less time than that.
In summary, it is clear that transition periods are short in comparison to timescales typical of the chemical reservoirs created during these transitions (average duration of 1.3·108 and 2.1·109 years, respectively). 5.2. The intensity of changesThe importance of transition periods for the chemical evolution of the universe was described in detail in Section 4, but it might be helpful to gather in one place the quantitative side of the discussion, i.e. the number of UDC classes describing phenomena that appear at a given stage of cosmic evolution (Table 1). The larger concentration of chemical evolution in transition periods than in phases is clear (average number of innovations per million years of 333 and 0.004, respectively). 5.3. Nucleation-like dynamicsAll transitions in the early universe are normally treated as phase transitions (Kibble 1982) and are assumed to involve nucleation and even ‘bubble formation’ (Liu et al. 1992). The localized initiation of all other phases of cosmic chemistry has already been described in Section 3. For instance, the ongoing transition from The Phase of Primitive Gas to the Phase of Enriched Gas takes place in individual stars and only later the high-metallicity gas with grains is spread throughout the galaxy. 5.4. The freeze-out of physical conditionsThe ‘freeze-out’ of certain physical conditions is best-known from those classical phase transitions which involve a symmetry break. For instance, during the crystallization of liquid water, a macroscopically isotropic liquid turns into an anisotropic solid, and the momentary configuration of water molecules at the point of nucleation determines the orientation of the ice crystal lattice. Which is especially important, once the optical axis has been ‘chosen’, it is stable and will not change unless dramatic physical processes occur. Similar processes are also discussed in cosmology (where momentary conditions of the Universe during phase transitions may have quite dramatic consequences, see e.g. Smolin 1995). This effect, applied to the evolution of cosmic chemistry, is probably best documented in the emergence of planetary chemistry and will be described in some detail in the following Section. It will be shown there that the momentary composition of the protoplanetary disk at the moment of the formation of a given planetary body will strongly determine its future chemical evolution. Because our knowledge of living systems is limited to a single example, we can only speculate on how much a slightly different composition of Earth at the moment of abiogenesis would change the chemistry of life (Bains 2004). There is no convincing evidence that the local state of interstellar matter during the planetary system formation influences its further evolution, although the injection of short-lived radioactive nuclides which dramatically influenced the evolution of Solar System bodies (Trieloff & Palme 2006, p. 77) may be thought of as a suggestive example of a process that contains a strong element of randomness. Also, the irreversible nature of the ‘choice’ is well illustrated by the relative isolation of planetary systems which will be discussed in the following Section. 6. The importance of planetary systems for chemical evolutionThe cosmological importance of planetary systems may seem difficult to assess, as we know in detail only one such system. To put the problem in a proper perspective, let us note that a recent evaluation of the Kepler data revealed that ca. 23% of all stars should harbor a close-in Earth-mass planet (Howard 2010), which gives a conservative number of 50 billion planetary systems similar to ours in the Milky Way alone. However, even our limited sample of one can yield valuable insights. Consider the following facts. First, let us note that the interstellar matter is relatively well-mixed (De Avillez & Mac Low 2008), so all planetary systems can be approximately treated as starting with a similar chemical composition. Also, because of the pressure of stellar winds and the shielding properties of heliospheric electromagnetic currents (Burgess 1997), planetary systems develop with little external influx of matter. Second, the early stages of planetary system evolution act to separate this initial homogeneous matter into a number of heterogeneous planetary-size reservoirs. This is mostly achieved by the orchestrated action of two groups of processes (Trieloff & Palme 2006). (1) Temperature gradients and gravitational forces fractionate elements and chemical compounds by condensation point and mass, respectively. This works both in protoplanetary disks (leading to the existence of metal- and rock-rich, gas-rich, and ice-rich sectors of Solar System) and later in individual planets (leading to the existence of metallic cores, rocky mantles, atmospheres, and cryospheres). (2) Gravitational interactions in the protoplanetary disk lead to the formation and migration of planets and their smaller companions. Third, these processes create a very diverse population of objects. In our Solar System, there are ca. 30-40 bodies large enough to have been rounded by gravity.[7] Even a cursory look at them reveals considerable diversity, even based on coarse orbital, thermodynamic, geophysical, or geomorphological criteria (see e.g. Taylor & McLennan 2008, Melosh 2011, Watters & Schultz 2012). Were there only a limited number of ways in which protoplanetary evolution can partition the solar nebula into planetary-size bits, we should expect duplicates in our sample of at least 30 such bodies. This is definitely not the case. Also, even our limited knowledge of extrasolar planetary systems shows that there are vast variations in their general architecture (Marcy et al. 2006), including the now confirmed discovery of planets orbiting binary systems (Doyle et al. 2011). Fourth, even tiny variations of such general planetary parameters translate to peculiar chemical phenomena. The colorful appearance of Jupiter’s Io, for instance, is caused by the temporary break-up of the familiar cyclo-octal sulfur compound S8 into unusual chain forms (Geissler 2003) which are highly unstable on Earth. For similar reasons related to elemental concentrations, a rich chlorine chemistry operates on Venus (Zhang et al. 2012). On Saturn’s Titan, the lack of atmospheric oxygen led to peculiar chemical phenomena, including the hypothesized oxygen-less biochemistry with familiar molecules having their ‘ammono analogs’, such as ‘ammono-ribose’ with amines instead of hydroxyls and a nitrogen heteroatom in the ring (Raulin & Owen 2003). Note that these local effects are not just curiosities of little cosmological importance. It is on the surfaces of the planets that a large part of the universe’s chemical creativity seems to unfold. Out of the 176 chemical phenomena studied here, 52 occur only in the context of planetary systems, and a more detailed analysis would almost certainly increase this proportion even further. The special case of life serves only to accentuate that point. Therefore, planetary systems may be seen as ‘atoms’ of cosmic chemical creativity: isolated laboratories where an initial homogeneous mixture is, through a highly efficient randomizing procedure, fractionated and subjected to various physical influences to yield a rich repertoire of chemical (and physical, mineralogical, geological…) phenomena. Based on what has been said above, it seems clear that our planetary system does not represent all of the cosmic chemical creativity. The 30% of all chemical phenomena studied here that are specific to planetary systems represents only a very limited sample of one, whereas spectroscopic observations of interstellar matter give us data from multiple spots, including extragalactic sources. To put it differently, an inclusion of only one planetary system to the census of chemical phenomena increased its size by a half – and there are literally billions of other planetary systems out there which may be reasonably suspected to host their own novel ‘chemistries’. If this perspective is valid, an increasing penetration of extrasolar planetary systems should bring a growing appreciation of the peculiar, local, and accidental nature of the chemistry that we know of. 7. ConclusionThe method described in this paper was designed to shed light on the complex relationships between chemistry and other natural sciences, but not in a static, ‘nomothetic’ (roughly: law-based) sense, but in the ‘idiographic’ (again, roughly: fact and event-based; for the source of this dichotomy, see: Windelband 1894) perspective of cosmic evolution. The evolution of galactic, stellar, planetary, and biotic systems proceeds with the concurrent evolution of chemical phenomena, and chemistry (understood as the totality of phenomena studies by chemical sciences) can be seen as a key element in the functioning of the cosmic organism, from its simple ‘embryonic’ stage to the present moment. It is vital to clearly understand the place of chemistry in natural sciences. Unfortunately, this issue is usually seen through the ‘static’ perspective of theoretical reductions, i.e. the reduction of chemistry to physics, the reduction of biology to chemistry, etc. Even a cursory look at the literature shows that the problem of reduction is still one of the major open issues in philosophy of chemistry (Vihalemm 2001, Earley 2004, Ostrovsky 2005, Scerri 2007, Boeyens 2008, p. vi.) and the reduction of chemistry to quantum mechanics seems to be one of the defining issues for the identity of the chemical sciences. This ‘nomothetic’ perspective, as I have already argued elsewhere (Lamza 2010), conceals the fact that chemistry seen as part of the ‘idiographic’ study of the history of the Universe is no more reducible to physics than physics to chemistry. In fact, this article intended to demonstrate that it is virtually impossible to understand the early Universe, the galaxies, stars, stellar envelopes, protoplanetary systems, or planets themselves without a proper appreciation for the growth of chemical complexity through time. From a ‘cosmo-historical’ perspective, the reduction of chemistry to physics is simply an absurd proposition; the most one could hope to prove is the reduction of a very tiny subset of the chemical sciences – the study of small molecular systems – to a very tiny subset of the physical sciences – the quantum theory; and even that is still controversial (see the references above). However, for this to be adequately appreciated, a very broad look at chemistry is needed. In philosophy of science chemistry is too often represented as a computational science concerned with calculating, to the nth-decimal place, the bond lengths and angles of small molecules. The dizzying richness of chemical phenomena – which includes colloids, flames, electrochemistry, and liquid crystals – is intimately intertwined with the richness of the evolving Cosmos, and in that perspective the traditional ‘big’ issue of intertheoretic reduction seems irrelevant and marginal for the identity of chemistry as part of the landscape of natural science. This led to the decision to use a library catalogue as a source of inspiration for describing ‘chemistry as such’ – a source which aims to contain a broad and rationally organized catalogue of all conceivable issues which are traditionally regarded as part of ‘chemistry’. I believe that there is a large and as yet unrecognized philosophical potential in library catalogues. After all, they are probably the best shot that humanity has had at compiling exhaustive and detailed lists of issues relevant for any conceivable topic – even if created without a particular philosophical agenda, with the praxis of cataloguing books as a stated goal. The UDC catalogue’s section 5 is in fact a well-thought, cleverly organized list of ‘all things that scientists write books about’ – and is thus as good a starting perspective on the subject of ‘all things chemical’ as one can hope for. This paper intends to demonstrate this potential in the study of the ‘cosmic history of chemical phenomena’ – where the assembled list of 176 chemical phenomena is not treated as a final word on ‘what chemistry is’, but as a starting point that should give the reader a feeling that at least a fair sample of topics in chemistry has been included. It should be noted that some subjects that might be considered parts of chemistry are outside the main ‘chemical’ UDC classes listed in section 2 (e.g. ‘550.4 Geochemistry’). Overall, it is clear that this method has limitations; after all, library catalogues are primarily created to help librarians, not to shed light on cosmic order. One good choice for future research of this point would be to try to supplement the list drawn from the UDC with a number of other sources, such as comprehensive lists of astromolecules, published classification of organic molecules, etc. Some philosophically interesting results that are systematically exposed by the method described in the article were already alluded to, such as:
That may be seen as another contribution to the unraveling of the complicated relationship between physics and chemistry, which is not limited to the reduction of chemistry to physics. Currently the inclusion of all 185 physics-related level-V UDC classes to the scheme described in this article is underway and it can be estimated that about 80% of physical processes denoted by those classes are directly causally linked to the preceding evolution of chemical phenomena.[8] A final advantage of the method may be now discussed: it naturally allows one to discuss the universe’s repertoire of chemical phenomena within a framework that is equally well suited for chemistry as it is for astrophysics, mineralogy, geology, or biology. This brings us even closer to see all natural sciences as a single science concerned with a single object – the evolving Cosmos. In fact, preliminary results show that the inclusion of further 717 level-V UDC classes representing the rest of the natural sciences shows considerable similarity of the patterns of cosmic evolution regardless of the type of system under consideration. Also, with a tool of that sort it can be shown in detail how physical, chemical, biological, and geological innovations co-occur throughout the past 3 billion years of our planet’s evolution (the great atmospheric oxidation event of 2.4 billion years ago being a particularly beautiful example) and what the place of chemical phenomena is in that process. Thus, chemical phenomena may be consistently described as part of the general architectonics of the universe, as exemplified by the use of the formalism of phases and phase transitions in Section 5. Thus, this ‘cosmohistorical’ perspective on chemical phenomena may make it easier to appreciate the ‘function’ of chemistry in the world, i.e. to try to understand what chemistry does as part of the evolving organism of the Cosmos, in unison with cosmological, astrophysical, geological, atmospheric, biological, or technological phenomena. AcknowledgementI would like to thank the referees for their constructive comments, and Prof. William White for his numerous helpful suggestions. Notes[1] It should be noted that this procedure – the transition from a list of fields of study to the list of natural phenomena – which in theory may sound tricky (and from a philosophical perspective means moving from epistemology to metaphysics), proved in practice to be immediate and straightforward. [2] Some authors (e.g. Vonlanthen et al. 2009) include microscopic amounts of even heavier elements suspected to be formed in the Big Bang Nucleosynthesis and calculate the abundances of molecules such as CH (relative abundance on the order of 10-19), NH (10-25) or HF (10-29), however this is speculative. [3] Although this is sometimes disputed and sterol synthesis has been implicated in some prokaryotic groups, notably actinobacteria (Donova 2007). [4] Spectroscopic signatures of homochirality are often suggested as a test for the presence of life in the context of astrobiology (Caglioti et al. 2006). [5] Although it might be formed during explosive nucleosynthesis in supernovae (Botvina & Mishustin 2010). [6] Where the word ‘transition’ is used to denote the period in which the matter’s ionization dropped from 99% to 1%. [7] It is a simple criterion used sometimes in planetary science to distinguish ‘small’ from ‘big’ bodies and appears currently in the IAU’s definition of a ‘planet’ (Soter 2006). [8] It is important to remember that the – admittedly very tenuous – quantitative flavor to this estimation is not intended as a demonstration of an objective measure, but rather as a stimulation to further discussion. ReferencesBains, W.: 2004, ‘Many chemistries could be used to build living systems’, Astrobiology, 4(2), 137-167. Bakes, E.L.O. & Tielens, A.G.G.M.: 1994, ‘The photoelectric heating mechanism for very small graphitic grains and polycyclic aromatic hydrocarbons’, The Astrophysical Journal, 427, 822-838. Botvina, A.S. & Mishustin, I.N.: 2010, ‘Statistical approach for supernova matter’, Nuclear Physics A, 843(1), 98-132. Boeyens, J.C.A.: 2008, Chemistry from First Principles, Berlin: Springer. Braunschweig, H.; Dewhurst, R.D.; Hammon, K.; Mies, J.; Radacki, K. & Vargas, A.: 2010, ‘Ambient-Temperature Isolation of a Compound with a Boron-Boron Triple Bond’, Science, 336(6087), 1420-1422. Burgess, D.: 1997, ‘Solar wind and interstellar medium coupling’, Solar and Heliospheric Plasma Physics, 489, 117-138. Caglioti, L.; Holczknecht, O.; Fujii, N.; Zucchi, C. & Gyula Palyi, G.: 2006, ‘Astrobiology and Biological Chirality’, Origins of Life and Evolution of Biospheres, 36(5-6), 459-466. Cavalier-Smith, T.: 1996, ‘A revised six-kingdom system of life’, Biological Reviews, 73, 203-266. Cody, G.D.; Heying, E.; Alexander, C.M.O.; Nittler, L.R.; Kilcoyne, A.L.D.; Sandford, S.A. & Stroud, R.M.: 2011, ‘Establishing a molecular relationship between chondritic and cometary organic solids’, Proceedings of the National Academy of Sciences, 108(48), 19171-19176. Cole, G.H. & Woolfson, M.M.: 2002, Planetary science: the science of planets around stars, Boca Raton: Taylor & Francis. Davis, A.M. & Richter, F.M: 2003, ‘Condensation and Evaporation of Solar System Materials’, in: Davis, A.M. (ed.): Treatise on Geochemistry. Volume 1: Meteorites, Comets, and Planets, Amsterdam et al.: Elsevier, pp. 406-430. De Avillez, M.A. & Mac Low, M.M.: 2008, ‘Mixing Timescales in a Supernova-driven Interstellar Medium’, The Astrophysical Journal, 581(2), 1047. Donova, M.V.: 2007, ‘Transformation of steroids by actinobacteria: A review’, Applied Biochemistry and Microbiology, 43(1), 1-14. Doyle, L.R.; Carter, J.A.; Fabrycky, D.C.; Slawson, R.W.; Howell, S.B.; Winn, J. et al.: 2001, ‘Kepler-16: a transiting circumbinary planet’, Science, 333(6049), 1602-1606. Dyson, F.J.: 1979, ‘Time without end: Physics and biology in an open universe’, Reviews of Modern Physics, 51(3), 447. Dyson, J.E., Williams, D.A.: 1997, The physics of the interstellar medium, Boca Raton: Taylor & Francis. Earley, J.E.: 2004, ‘Would Introductory Chemistry Courses Work Better with a New Philosophical Basis?’, Foundations of Chemistry, 6, 137-160. Fukugita, M.; Hogan, C.J. & Peebles, P.J.E.: 1998, ‘The Cosmic Baryon Budget’, Astrophysical Journal, 503, 518-530. Fukugita, M. & Peebles, P.J.E.: 2004, The Cosmic Energy Inventory, Astrophysical Journal, 616, 643-668. Gaur, V.P.; Tripathi, B.M.; Joshi, G.C. & Pande, M.C.: 1988, ‘Molecules in white dwarfs’, Astrophysics and Space Science, 147(1), 107-113. Geissler, P.E.: 2003, ‘Volcanic activity on Io during the Galileo era’, Annual Review of Earth and Planetary Sciences, 31, 175-211. Gribble, G.W.: 1994, ‘The natural production of chlorinated compounds’, Environmental Science & Technology, 28(7), 310A-379A. Haken, H.: 1978, Synergetics. An Introduction. Nonequilibrium Phase Transitions and Self-Organization in Physics, Chemistry and Biology, 2nd edn., Heidelberg: Springer. Hazen, R.M.; Papineau, D.; Bleeker, W.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A. & Yang, H.: 2008, ‘Mineral evolution’, American Mineralogist, 93, 1693-1720. Herbst, E.: 2001, ‘The chemistry of interstellar space’, Chemical Society Reviews, 30, 168-176. Herwig, F.: 2005, ‘Evolution of asymptotic giant branch stars’, Annual Review of Astronomy and Astrophysics, 43, 435-479. Howard, A.W.; Marcy, G.W.; Johnson, J.A.; Fischer, D.A.; Wright, J.T.; Isaacson, H., et al.: 2010, ‘The occurrence and mass distribution of close-in super-Earths, Neptunes, and Jupiters’, Science, 330(6004), 653-655. Jones, A.P.: 2004, ‘Dust destruction processes’, Astrophysics of Dust. ASP Conference Series, 309, 347-367. Kaufmann, S.: 1996, At Home in the Universe, Oxford: Oxford University Press. Kibble, T.W.B.: 1982, ‘Phase transitions in the early universe’, Acta Physica Polonica B, 13(10-11), 723. Koga, N.; Tatsumi-Koga, R.; Liu, G.; Xiao, R.; Acton, T.B.; Montelione, G.T. & Baker, D.: 2012, ‘Principles for designing ideal protein structures’, Nature, 491, 222-229. Krasovitski, B.; Frenkel, V.; Shoham, S. & Kimmel, E.: 2011, ‘Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects’, Proceedings of the National Academy of Sciences, 108(8), 3258-3263. Lamza, L.: 2010, ‘How much history can chemistry take?’, Hyle: International Journal for Philosophy of Chemistry, 16(2), 104-120. Lange, K.K.; Tellgren, E.I.; Hoffmann, M.R. & Helgaker, T.: 2012, ‘A paramagnetic bonding mechanism for diatomics in strong magnetic fields’, Science, 337(6092), 327-331. Lattimer, J.M.; Schramm, D.N. & Grossman, L.: 1978, ‘Condensation in supernova ejecta and isotopic anomalies in meteorites’, The Astrophysical Journal, 219, 230-249. Liddle, A.: 2003, An Introduction to Modern Cosmology, 2nd edn., Weinheim: Wiley-VCH. Liu, B. H.; McLerran, L. & Turok, N.: 1992, ‘Bubble nucleation and growth at a baryon-number-producing electroweak phase transition’, Physical Review D, 46(6), 2668. Maoli, R.; Melchiorri, F. & Tosti, D.: 1994, ‘Molecules in the postrecombination universe and microwave background anisotropies’, Astrophysical Journal, Part 1, 425(2), 372-381. Marcy, G.; Fischer, D.A.; Butler, R.P. & Vogt, S.S.: 2006, ‘Properties of exoplanets: a Doppler study of 1330 stars’, in: Klahr, H. & Brandner, W. (eds.), Planet Formation, Cambridge: Cambridge University Press, pp. 179-191. Martins, Z.; Watson, J.S.; Sephton, M.A.; Botta, O.; Ehrenfreund, P. & Gilmour, I.: 2006, ‘Free dicarboxylic and aromatic acids in the carbonaceous chondrites Murchison and Orgueil’, Meteoritics & Planetary Science, 41(7), 1073-1080. Melosh, H.J.: 2011, Planetary Surface Processes, Cambridge: Cambridge University Press. Morita, T.; Bulanov, A.V.; Esirkepov, T.Z.; Koga, J. & Kando, M.: 2012, ‘Proton Acceleration due to Anisotropic Coulomb Explosion of a Double-Layer Target Irradiated by an Intense Laser Pulse’, Journal of the Physical Society of Japan, 81, 024501. Ostrovsky, V.N.: 2005, ‘Towards a Philosophy of Approximations in the "Exact" Sciences’, Hyle: International Journal for Philosophy of Chemistry, 11(2), 101-126. Pagel, B.E.J.: 1997, Nucleosynthesis and Chemical Evolution of Galaxies, Cambridge: Cambridge University Press. Palme, H. & Jones, A.: 2003, ‘Solar System Abundances of the Elements’, in: Davis, A.M. (ed.), Treatise on Geochemistry. Volume 1: Meteorites, Comets, and Planets, Amsterdam et al.: Elsevier, pp. 41-61. Pilling, S.; Andrade, D.P.P.; da Silveira, E.F.; Rothard, H.; Domaracka, A. & Boduch, P.: 2012, ‘Formation of unsaturated hydrocarbons in interstellar ice analogues by cosmic rays’, Monthly Notices of the Royal Astronomical Society, 423(3), 2209-2221. Prigogine, I. & Stengers. I.: 1984, Order out of Chaos. Man’s New Dialogue with Nature, London: Flamingo. Raulin, F.; Owen, T.: 2003, ‘Organic chemistry and exobiology on Titan’, in: Russell, C.T. (ed.): 2003, The Cassini-Huygens Mission, Amsterdam: Springer, pp. 377-394. Rees, M.: 2003, Our Final Hour, New York: Basic Books. Remusat, L.; Derenne, S.; Robert, F. & Knicker, H.: 2005, ‘New pyrolytic and spectroscopic data on Orgueil and Murchison insoluble organic matter: A different origin than soluble?’, Geochimica et Cosmochimica Acta, 69(15), 3919-3932. Rohde, R.A. & Muller, R.A.: 2005, ‘Cycles in fossil diversity’, Nature, 434, 208-210. Scerri, E.R.: 2007, ‘The Ambiguity of Reduction’, Hyle: International Journal for Philosophy of Chemistry, 13(2), 67-81. Schneider, E.D. & Sagan, D.: 2006, Into the Cool: Energy Flow, Thermodynamics, and Life, Chicago: University of Chicago Press. Shaw, A.M.: 2006, Astrochemistry: From Astronomy to Astrobiology, Chichester: Wiley. Simcoe, R.A; Sullivan, P.W.; Cooksey, K.L.; Kao, M.M.; Matejek, M.S. & Burgasser, A.J.: 2012, ‘Extremely metal-poor gas at a redshift of 7’, Nature, 492, 79–82. Sinha, K.: 1991, ‘Molecules in the Sun’, Proceedings of the Astronomical Society of Australia, 9(1), 32-36. Smolin, L.: 1995, ‘Cosmology as a problem in critical phenomena’, in: R. López-Peña et al. (eds.), Complex systems and binary networks, Heidelberg: Springer, pp. 184-223. Soter, S.: 2006, ‘What is a Planet?’, The Astronomical Journal, 132(6), 2513. Stuhl, B.K.; Hummon, M.T.; Yeo, M.; Quemener, G.; Bohn, J.L. & Ye, J.: 2012, ‘Evaporative cooling of the dipolar hydroxyl radical’, Nature, 492, 396-400. Taylor, S.R. & McLennan, S.: 2008, Planetary Crusts, Cambridge: Cambridge University Press. Tielens, A.G.G.M.: 2010, The Physics and Chemistry of Interstellar Medium, Cambridge: Cambridge University Press. Tolstikhin, I. & Kramers, J.: 2008, The evolution of matter: from the big bang to the present day, Cambridge: Cambridge University Press. Trieloff, M. & Palme, H.: 2006, ‘The origin of solids in the early Solar System’, in: Klahr, H., Brandner, W. (eds.), Planet Formation, Cambridge: Cambridge University Press, pp. 64-89. UDC: 2005, Universal Decimal Classification. Standard Edition, London: British Standards Institution. Valentine, J.W.: 2004, On the origin of phyla, Chicago: University of Chicago Press. Vihalemm, R.: 2001, ‘Chemistry as an Interesting Subject for the Philosophy of Science’, in: Vihalemm, R. (ed.): Estonian Studies in the History and Philosophy of Science (Boston Studies in the Philosophy of Science, vol. 219), Dordrecht: Kluwer, pp. 185-200. Vonlanthen, P.; Rauscher, T.; Winteler, C.; Puy, D.; Signore, M. & Dubrovich, V.: 2009, ‘Chemistry of heavy elements in the Dark Ages’, Astronomy and Astrophysics, 503(1), 47-59. Wacey, D.; Kilburn, M.R.; Saunders, M.; Cliff, J. & Brasier, M.D.: 2011, ‘Microfossils of sulphur-metabolizing cells in 3.4-billion-year-old rocks of Western Australia’, Nature Geoscience, 4(10), 698-702. Wang, Y.; Wang, Y.; Breed, D.R.; Manoharan, V.N.; Feng, L.; Hollingsworth, A.D.; Weck, M. & Pine, D.J.: 2012, ‘Colloids with valence and specific directional bonding’, Nature, 491, 51–55. Watters, T.R. & Schultz, R.A.: 2012, Planetary Tectonics, Cambridge: Cambridge University Press. Whittet, D.C.B (ed.): 1997, Planetary and Interstellar Processes Relevant to the Origins of Life, Dordrecht: Kluwer. Williams, Q.: 2007, ‘Water, the Solid Earth, and the Atmosphere: The Genesis and Effects of a Wet Surface on a Mostly Dry Planet’, in: Stevenson, D. (ed.), Treatise on Geophysics. Volume 9. Evolution of the Earth, Amsterdam et al.: Elsevier, pp. 121-144. Windelband, W: 1894, Geschichte und Naturwissenschaft, Strassburg: Heitz. Zhang, X.; Liang, M.C.; Mills, F.P.; Belyaev, D.A. & Yung, Y.L.: 2012, ‘Sulfur chemistry in the middle atmosphere of Venus’, Icarus, 217(2), 714-739. Lukasz Lamza: |